Multi-stage delivery nano-particle systems for therapeutic applications
- PMID: 20493927
- PMCID: PMC2948075
- DOI: 10.1016/j.bbagen.2010.05.004
Multi-stage delivery nano-particle systems for therapeutic applications
Abstract
Background: The daunting task for drug molecules to reach pathological lesions has fueled rapid advances in Nanomedicine. The progressive evolution of nanovectors has led to the development of multi-stage delivery systems aimed at overcoming the numerous obstacles encountered by nanovectors on their journey to the target site.
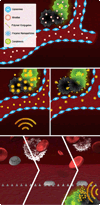
Scope of review: This review summarizes major findings with respect to silicon-based drug delivery vectors for cancer therapeutics and imaging. Based on rational design, well-established silicon technologies have been adapted for the fabrication of nanovectors with specific shapes, sizes, and porosities. These vectors are part of a multi-stage delivery system that contains multiple nano-components, each designed to achieve a specific task with the common goal of site-directed delivery of therapeutics.
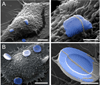
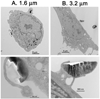
Major conclusions: Quasi-hemispherical and discoidal silicon microparticles are superior to spherical particles with respect to margination in the blood, with particles of different shapes and sizes having unique distributions in vivo. Cellular adhesion and internalization of silicon microparticles is influenced by microparticle shape and surface charge, with the latter dictating binding of serum opsonins. Based on in vitro cell studies, the internalization of porous silicon microparticles by endothelial cells and macrophages is compatible with cellular morphology, intracellular trafficking, mitosis, cell cycle progression, cytokine release, and cell viability. In vivo studies support superior therapeutic efficacy of liposomal encapsulated siRNA when delivered in multi-stage systems compared to free nanoparticles. This article is part of a Special Issue entitled Nanotechnologies - Emerging Applications in Biomedicine.
2010 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Mitotic trafficking of silicon microparticles.Nanoscale. 2009 Nov;1(2):250-9. doi: 10.1039/b9nr00138g. Epub 2009 Oct 5. Nanoscale. 2009. PMID: 20644846 Free PMC article.
-
Engineering multi-stage nanovectors for controlled degradation and tunable release kinetics.Biomaterials. 2013 Nov;34(33):8469-77. doi: 10.1016/j.biomaterials.2013.07.049. Epub 2013 Jul 30. Biomaterials. 2013. PMID: 23911070 Free PMC article.
-
Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics.Acc Chem Res. 2011 Oct 18;44(10):979-89. doi: 10.1021/ar200077p. Epub 2011 Sep 8. Acc Chem Res. 2011. PMID: 21902173 Free PMC article. Review.
-
Cellular association and assembly of a multistage delivery system.Small. 2010 Jun 21;6(12):1329-40. doi: 10.1002/smll.201000126. Small. 2010. PMID: 20517877 Free PMC article.
-
Porous silicon advances in drug delivery and immunotherapy.Curr Opin Pharmacol. 2013 Oct;13(5):834-41. doi: 10.1016/j.coph.2013.06.006. Epub 2013 Jul 8. Curr Opin Pharmacol. 2013. PMID: 23845260 Free PMC article. Review.
Cited by
-
Hyperpolarized MRI with silicon micro and nanoparticles: Principles and applications.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 Nov;13(6):e1722. doi: 10.1002/wnan.1722. Epub 2021 May 13. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 33982426 Free PMC article. Review.
-
Enhanced gene delivery in porcine vasculature tissue following incorporation of adeno-associated virus nanoparticles into porous silicon microparticles.J Control Release. 2014 Nov 28;194:113-21. doi: 10.1016/j.jconrel.2014.08.020. Epub 2014 Aug 30. J Control Release. 2014. PMID: 25180449 Free PMC article.
-
Redirecting Transport of Nanoparticle Albumin-Bound Paclitaxel to Macrophages Enhances Therapeutic Efficacy against Liver Metastases.Cancer Res. 2016 Jan 15;76(2):429-39. doi: 10.1158/0008-5472.CAN-15-1576. Epub 2016 Jan 7. Cancer Res. 2016. PMID: 26744528 Free PMC article.
-
Multifunctional iron platinum stealth immunomicelles: targeted detection of human prostate cancer cells using both fluorescence and magnetic resonance imaging.J Nanopart Res. 2011 Oct 1;13(10):4717-4729. doi: 10.1007/s11051-011-0439-3. J Nanopart Res. 2011. PMID: 22121333 Free PMC article.
-
Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice.PLoS One. 2013 Apr 23;8(4):e61646. doi: 10.1371/journal.pone.0061646. Print 2013. PLoS One. 2013. PMID: 23626707 Free PMC article.
References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. - PubMed
-
- Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570–576. - PubMed
-
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

