Current understanding of the mechanism of HPV infection
- PMID: 20494219
- PMCID: PMC3493113
- DOI: 10.1016/j.ygyno.2010.04.004
Current understanding of the mechanism of HPV infection
Abstract
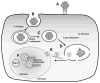
HPVs (human papillomaviruses) and other papillomaviruses have a unique mechanism of infection that has likely evolved to limit infection to the basal cells of stratified epithelium, the only tissue in which they replicate. Recent studies in a mouse cervicovaginal challenge model indicate that, surprisingly, the virus cannot initially bind to keratinocytes in vivo. Rather it must first bind via its L1 major capsid protein to heparan sulfate proteoglycans (HSPGs) on segments of the basement membrane (BM) exposed after epithelial trauma and undergo a conformational change that exposes the N-terminus of L2 minor capsid protein to furin cleavage. L2 proteolysis exposes a previously occluded surface of L1 that binds an as yet undetermined cell surface receptor on keratinocytes that have migrated over the BM to close the wound. Papillomaviruses are the only viruses that are known to initiate their infectious process at an extracellular site. In contrast to the in vivo situation, the virions can bind directly to many cultured cell lines through cell surface HSPGs and then undergo a similar conformational change and L2 cleavage. Transfer to the secondary receptor leads to internalization, uncoating in late endosomes, escape from the endosome by an L2-dependent mechanism, and eventual trafficking of an L2-genome complex to specific subnuclear domains designated ND10 bodies, where viral gene transcription is initiated. The infectious process is remarkably slow and asynchronous both in vivo and in cultured cells, taking 12-24h for initiation of transcription. The extended exposure of antibody neutralizing determinants while the virions reside on the BM and cell surfaces might, in part, account for the remarkable effectiveness of vaccines based on neutralizing antibodies to L1 virus-like particles or the domain of L2 exposed after furin cleavage.
Copyright (c) 2010. Published by Elsevier Inc.
Conflict of interest statement
JTS is inventor on US-government-owned patents licensed to Merck and GlaxoSmithKline and entitled to limited royalties from these patents.
PMD does not have a conflict of interest.
RCK does not have a conflict of interest.
Figures
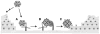
References
-
- Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–3. - PubMed
-
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

