Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation
- PMID: 20494516
- PMCID: PMC2916061
- DOI: 10.1016/j.pain.2010.04.001
Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation
Abstract
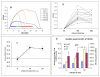
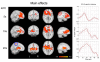
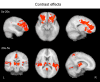
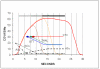
Noxious cutaneous contact heat stimuli (48 degrees C) are perceived as increasingly painful when the stimulus duration is extended from 5 to 10s, reflecting the temporal summation of central neuronal activity mediating heat pain. However, the sensation of increasing heat pain disappears, reaching a plateau as stimulus duration increases from 10 to 20s. We used functional magnetic resonance imaging (fMRI) in 10 healthy subjects to determine if active central mechanisms could contribute to this psychophysical plateau. During heat pain durations ranging from 5 to 20s, activation intensities in the bilateral orbitofrontal cortices and the activation volume in the left primary (S1) somatosensory cortex correlated only with perceived stimulus intensity and not with stimulus duration. Activation volumes increased with both stimulus duration and perceived intensity in the left lateral thalamus, posterior insula, inferior parietal cortex, and hippocampus. In contrast, during the psychophysical plateau, both the intensity and volume of thalamic and cortical activations in the right medial thalamus, right posterior insula, and left secondary (S2) somatosensory cortex continued to increase with stimulus duration but not with perceived stimulus intensity. Activation volumes in the left medial and right lateral thalamus, and the bilateral mid-anterior cingulate, left orbitofrontal, and right S2 cortices also increased only with stimulus duration. The increased activity of specific thalamic and cortical structures as stimulus duration, but not perceived intensity, increases is consistent with the recruitment of a thalamocortical mechanism that participates in the modulation of pain-related cortical responses and the temporal summation of heat pain.
Published by Elsevier B.V.
Figures


Similar articles
-
Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain.J Neurophysiol. 1996 Jul;76(1):571-81. doi: 10.1152/jn.1996.76.1.571. J Neurophysiol. 1996. PMID: 8836245 Clinical Trial.
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
-
BOLD responses in somatosensory cortices better reflect heat sensation than pain.J Neurosci. 2012 Apr 25;32(17):6024-31. doi: 10.1523/JNEUROSCI.0006-12.2012. J Neurosci. 2012. PMID: 22539862 Free PMC article.
-
Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography.J Neurophysiol. 2001 Feb;85(2):951-9. doi: 10.1152/jn.2001.85.2.951. J Neurophysiol. 2001. PMID: 11160525
-
Central mechanisms of pain perception.Suppl Clin Neurophysiol. 2004;57:39-49. doi: 10.1016/s1567-424x(09)70341-1. Suppl Clin Neurophysiol. 2004. PMID: 16106604 Review.
Cited by
-
Developing an optimized strategy with transcranial direct current stimulation to enhance the endogenous pain control system in fibromyalgia.Expert Rev Med Devices. 2018 Dec;15(12):863-873. doi: 10.1080/17434440.2018.1551129. Epub 2018 Dec 3. Expert Rev Med Devices. 2018. PMID: 30501532 Free PMC article. Review.
-
The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction.Arthritis Rheumatol. 2015 May;67(5):1395-1405. doi: 10.1002/art.39043. Arthritis Rheumatol. 2015. PMID: 25622796 Free PMC article.
-
Regional brain functions in the resting state indicative of potential differences between depression and chronic pain.Sci Rep. 2017 Jun 7;7(1):3003. doi: 10.1038/s41598-017-03522-1. Sci Rep. 2017. PMID: 28592893 Free PMC article.
-
Comparing neural responses to cutaneous heat and pressure pain in healthy participants.Sci Rep. 2025 Apr 24;15(1):14387. doi: 10.1038/s41598-025-99247-7. Sci Rep. 2025. PMID: 40274927 Free PMC article.
-
Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia.J Pain. 2012 Feb;13(2):195-206. doi: 10.1016/j.jpain.2011.11.001. Epub 2012 Jan 13. J Pain. 2012. PMID: 22245361 Free PMC article. Clinical Trial.
References
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. - PubMed
-
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: A marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. - PubMed
-
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. - PubMed
-
- Beitel RE, Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey’s face. J Neurophysiol. 1976;39:1160–1175. - PubMed
-
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

