Acupuncture, the limbic system, and the anticorrelated networks of the brain
- PMID: 20494627
- PMCID: PMC3754836
- DOI: 10.1016/j.autneu.2010.03.022
Acupuncture, the limbic system, and the anticorrelated networks of the brain
Abstract
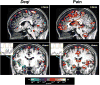
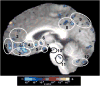
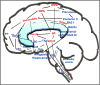
The study of the mechanism of acupuncture action was revolutionized by the use of functional magnetic resonance imaging (fMRI). Over the past decade, our fMRI studies of healthy subjects have contributed substantially to elucidating the central effect of acupuncture on the human brain. These studies have shown that acupuncture stimulation, when associated with sensations comprising deqi, evokes deactivation of a limbic-paralimbic-neocortical network, which encompasses the limbic system, as well as activation of somatosensory brain regions. These networks closely match the default mode network and the anti-correlated task-positive network described in the literature. We have also shown that the effect of acupuncture on the brain is integrated at multiple levels, down to the brainstem and cerebellum. Our studies support the hypothesis that the effect of acupuncture on the brain goes beyond the effect of attention on the default mode network or the somatosensory stimulation of acupuncture needling. The amygdala and hypothalamus, in particular, show decreased activation during acupuncture stimulation that is not commonly associated with default mode network activity. At the same time, our research shows that acupuncture stimulation needs to be done carefully, limiting stimulation when the resulting sensations are very strong or when sharp pain is elicited. When acupuncture induced sharp pain, our studies show that the deactivation was attenuated or reversed in direction. Our results suggest that acupuncture mobilizes the functionally anti-correlated networks of the brain to mediate its actions, and that the effect is dependent on the psychophysical response. In this work we also discuss multiple avenues of future research, including the role of neurotransmitters, the effect of different acupuncture techniques, and the potential clinical application of our research findings to disease states including chronic pain, major depression, schizophrenia, autism, and Alzheimer's disease.
Published by Elsevier B.V.
Figures




References
-
- Bai L, Qin W, Tian J, Dong M, Pan X, Chen P, Dai J, Yang W, Liu Y. Acupuncture modulates spontaneous activities in the anti-correlated resting brain networks. Brain Res. 2009;1279:37–49. - PubMed
-
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite A, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. - PubMed
-
- Benes FM. Neural circuitry models of schizophrenia: is it dopamine, GABA, glutamate, or something else? Biol Psychiatry. 2009;65:1003–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21-AT00978/AT/NCCIH NIH HHS/United States
- K01-AT-002166-01/AT/NCCIH NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- 1-P01-AT002048-01/AT/NCCIH NIH HHS/United States
- R21 AT000978/AT/NCCIH NIH HHS/United States
- F05 AT003770/AT/NCCIH NIH HHS/United States
- F05-AT003770/AT/NCCIH NIH HHS/United States
- 2-P01-AT002048-06/AT/NCCIH NIH HHS/United States
- P41RR14075/RR/NCRR NIH HHS/United States
- K01 AT002166/AT/NCCIH NIH HHS/United States
- P01 AT002048/AT/NCCIH NIH HHS/United States
- NS34189/NS/NINDS NIH HHS/United States
- R01 NS034189/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

