Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial
- PMID: 20494931
- PMCID: PMC2948102
- DOI: 10.3324/haematol.2009.019182
Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial
Abstract
Background: Allogeneic stem cell transplantation is usually considered the only curative treatment option for patients with advanced or transformed myelodysplastic syndromes in complete remission, but post-remission chemotherapy and autologous stem cell transplantation are potential alternatives, especially in patients over 45 years old.
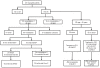
Design and methods: We evaluated, after intensive anti-leukemic remission-induction chemotherapy, the impact of the availability of an HLA-identical sibling donor on an intention-to treat basis. Additionally, all patients without a sibling donor in complete remission after the first consolidation course were randomized to either autologous peripheral blood stem cell transplantation or a second consolidation course consisting of high-dose cytarabine.
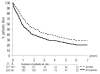
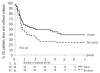
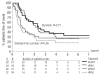
Results: The 4-year survival of the 341 evaluable patients was 28%. After achieving complete remission, the 4-year survival rates of patients under 55 years old with or without a donor were 54% and 41%, respectively, with an adjusted hazard ratio of 0.81 (95% confidence interval [95% CI], 0.49-1.35) for survival and of 0.67 (95% CI, 0.42-1.06) for disease-free survival. In patients with intermediate/high risk cytogenetic abnormalities the hazard ratio in multivariate analysis was 0.58 (99% CI, 0.22-1.50) (P=0.14) for survival and 0.46 (99% CI, 0.22-1.50) for disease-free survival (P=0.03). In contrast, in patients with low risk cytogenetic characteristics the hazard ratio for survival was 1.17 (99% CI, 0.40-3.42) and that for disease-free survival was 1.02 (99% CI, 0.40-2.56). The 4-year survival of the 65 patients randomized to autologous peripheral blood stem cell transplantation or a second consolidation course of high-dose cytarabine was 37% and 27%, respectively. The hazard ratio in multivariate analysis was 1.22 (95% CI, 0.65-2.27) for survival and 1.02 (95% CI, 0.56-1.85) for disease-free survival.
Conclusions: Patients with a donor and candidates for allogeneic stem cell transplantation in first complete remission may have a better disease-free survival than those without a donor in case of myelodysplastic syndromes with intermediate/high-risk cytogenetics. Autologous peripheral blood stem cell transplantation does not provide longer survival than intensive chemotherapy.
Trial registration: ClinicalTrials.gov NCT00002926.
Figures
Comment in
-
IDH1 and IDH2 mutations in myeloid neoplasms--novel paradigms and clinical implications.Haematologica. 2010 Oct;95(10):1623-7. doi: 10.3324/haematol.2010.030015. Haematologica. 2010. PMID: 20884716 Free PMC article. No abstract available.
References
-
- Mufti GJ, Stevens JR, Oscier DG, Hamblin TJ, Machin D. Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol. 1985;59(3):425–33. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. - PubMed
-
- Maes B, Meeus P, Michaux L, Bijnens L, Boogaerts M, Hagemeijer A, et al. Application of the International Prognostic Scoring System for myelodysplastic syndromes. Ann Oncol. 1999;10(7):825–9. - PubMed
-
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13(2):193–207. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- 5U10 CA11488-32/CA/NCI NIH HHS/United States
- 5U10 CA11488-27/CA/NCI NIH HHS/United States
- 5U10 CA11488-38/CA/NCI NIH HHS/United States
- 5U10 CA11488-28/CA/NCI NIH HHS/United States
- 5U10 CA11488-35/CA/NCI NIH HHS/United States
- 5U10 CA11488-37/CA/NCI NIH HHS/United States
- U10 CA011488/CA/NCI NIH HHS/United States
- 5U10 CA11488-29/CA/NCI NIH HHS/United States
- 5U10 CA11488-34/CA/NCI NIH HHS/United States
- 5U10 CA11488-30/CA/NCI NIH HHS/United States
- 5U10 CA11488-31/CA/NCI NIH HHS/United States
- 5U10 CA11488-26/CA/NCI NIH HHS/United States
- 5U10 CA11488-36/CA/NCI NIH HHS/United States
- 5U10 CA11488-33/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

