Reactive oxygen species, Ki-Ras, and mitochondrial superoxide dismutase cooperate in nerve growth factor-induced differentiation of PC12 cells
- PMID: 20495008
- PMCID: PMC2911279
- DOI: 10.1074/jbc.M109.098525
Reactive oxygen species, Ki-Ras, and mitochondrial superoxide dismutase cooperate in nerve growth factor-induced differentiation of PC12 cells
Abstract
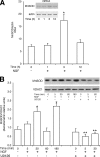
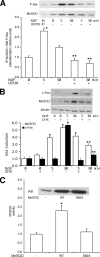
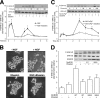
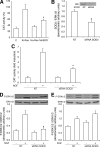
Nerve growth factor (NGF) induces terminal differentiation in PC12, a pheochromocytoma-derived cell line. NGF binds a specific receptor on the membrane and triggers the ERK1/2 cascade, which stimulates the transcription of neural genes. We report that NGF significantly affects mitochondrial metabolism by reducing mitochondrial-produced reactive oxygen species and stabilizing the electrochemical gradient. This is accomplished by stimulation of mitochondrial manganese superoxide dismutase (MnSOD) both transcriptionally and post-transcriptionally via Ki-Ras and ERK1/2. Activation of MnSOD is essential for completion of neuronal differentiation because 1) expression of MnSOD induces the transcription of a neuronal specific promoter and neurite outgrowth, 2) silencing of endogenous MnSOD by small interfering RNA significantly reduces transcription induced by NGF, and 3) a Ki-Ras mutant in the polylysine stretch at the COOH terminus, unable to stimulate MnSOD, fails to induce complete differentiation. Overexpression of MnSOD restores differentiation in cells expressing this mutant. ERK1/2 is also downstream of MnSOD, as a SOD mimetic drug stimulates ERK1/2 with the same kinetics of NGF and silencing of MnSOD reduces NGF-induced late ERK1/2. Long term activation of ERK1/2 by NGF requires SOD activation, low levels of hydrogen peroxide, and the integrity of the microtubular cytoskeleton. Confocal immunofluorescence shows that NGF stimulates the formation of a complex containing membrane-bound Ki-Ras, microtubules, and mitochondria. We propose that active NGF receptor induces association of mitochondria with plasma membrane. Local activation of ERK1/2 by Ki-Ras stimulates mitochondrial SOD, which reduces reactive oxygen species and produces H(2)O(2). Low and spatially restricted levels of H(2)O(2) induce and maintain long term ERK1/2 activity and ultimately differentiation of PC12 cells.
Figures






References
-
- Santillo M., Mondola P., Serù R., Annella T., Cassano S., Ciullo I., Tecce M. F., Iacomino G., Damiano S., Cuda G., Paternò R., Martignetti V., Mele E., Feliciello A., Avvedimento E. V. (2001) Curr. Biol. 11, 614–619 - PubMed
-
- Cuda G., Paternò R., Ceravolo R., Candigliota M., Perrotti N., Perticone F., Faniello M. C., Schepis F., Ruocco A., Mele E., Cassano S., Bifulco M., Santillo M., Avvedimento E. V. (2002) Circulation 105, 968–974 - PubMed
-
- Suzukawa K., Miura K., Mitsushita J., Resau J., Hirose K., Crystal R., Kamata T. (2000) J. Biol. Chem. 275, 13175–13178 - PubMed
-
- Ibi M., Katsuyama M., Fan C., Iwata K., Nishinaka T., Yokoyama T., Yabe-Nishimura C. (2006) Free Radic. Biol. Med. 40, 1785–1795 - PubMed
-
- Sampath D., Jackson G. R., Werrbach-Perez K., Perez-Polo J. R. (1994) J. Neurochem. 62, 2476–2479 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

