Absence of the inhibitory G-protein Galphai2 predisposes to ventricular cardiac arrhythmia
- PMID: 20495013
- PMCID: PMC3401367
- DOI: 10.1161/CIRCEP.109.894329
Absence of the inhibitory G-protein Galphai2 predisposes to ventricular cardiac arrhythmia
Abstract
Background: We explored the role that inhibitory heterotrimeric G-proteins play in ventricular arrhythmia.
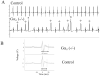
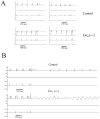
Methods and results: Mice with global genetic deletion of Galpha(i2) [Galpha(i2) (-/-)] were studied and found, based on telemetry, to have a prolonged QT interval on surface ECG when awake. In vivo electrophysiology studies revealed that the Galpha(i2) (-/-) mice have a reduced ventricular effective refractory period and a predisposition to ventricular tachycardia when challenged with programmed electrical stimulation. Neither control nor combined global deletion of Galpha(i1) and Galpha(i3) mice showed these abnormalities. There was no evidence for structural heart disease at this time point in the Galpha(i2) (-/-) mice as assessed by cardiac histology and echocardiography. The absence of Galpha(i2) thus leads to a primary electrical abnormality, and we explored the basis for this finding. With patch clamping, single isolated ventricular cells showed that Galpha(i2) (-/-) mice had a prolonged ventricular action potential duration (APD) but steeper action potential shortening as the diastolic interval was reduced in restitution studies. Gene expression studies showed increased expression of L-type Ca(2+) channel subunits, and patch clamping revealed an increase in these currents in Galpha(i2) (-/-) mice. There were no changes in K(+) currents.
Conclusions: The absence of inhibitory G-protein signaling mediated through Galpha(i2) is a substrate for ventricular arrhythmias.
Conflict of interest statement
Figures



Similar articles
-
Gαi2- and Gαi3-specific regulation of voltage-dependent L-type calcium channels in cardiomyocytes.PLoS One. 2011;6(9):e24979. doi: 10.1371/journal.pone.0024979. Epub 2011 Sep 26. PLoS One. 2011. PMID: 21966394 Free PMC article.
-
Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes.Circ Res. 2000 Nov 10;87(10):903-9. doi: 10.1161/01.res.87.10.903. Circ Res. 2000. PMID: 11073886
-
Remodeling of repolarization and arrhythmia susceptibility in a myosin-binding protein C knockout mouse model.Am J Physiol Heart Circ Physiol. 2017 Sep 1;313(3):H620-H630. doi: 10.1152/ajpheart.00167.2017. Epub 2017 Jun 23. Am J Physiol Heart Circ Physiol. 2017. PMID: 28646025 Free PMC article.
-
Differential effects of inhibitory G protein isoforms on G protein-gated inwardly rectifying K+ currents in adult murine atria.Am J Physiol Cell Physiol. 2018 May 1;314(5):C616-C626. doi: 10.1152/ajpcell.00271.2016. Epub 2018 Jan 17. Am J Physiol Cell Physiol. 2018. PMID: 29342363 Free PMC article.
-
Defective macrophage migration in Gαi2- but not Gαi3-deficient mice.J Immunol. 2012 Jul 15;189(2):980-7. doi: 10.4049/jimmunol.1200891. Epub 2012 Jun 15. J Immunol. 2012. PMID: 22706085
Cited by
-
Circulating Cell and Plasma microRNA Profiles Differ between Non-ST-Segment and ST-Segment-Elevation Myocardial Infarction.Fam Med Med Sci Res. 2013 Oct 1;2(2):108. doi: 10.4172/2327-4972.1000108. Fam Med Med Sci Res. 2013. PMID: 24432306 Free PMC article.
-
The Role of Inhibitory G Proteins and Regulators of G Protein Signaling in the in vivo Control of Heart Rate and Predisposition to Cardiac Arrhythmias.Front Physiol. 2012 Apr 24;3:96. doi: 10.3389/fphys.2012.00096. eCollection 2012. Front Physiol. 2012. PMID: 22783193 Free PMC article.
-
Analyses of Gnai3-iresGFP reporter mice reveal unknown Gαi3 expression sites.Sci Rep. 2021 Jul 12;11(1):14271. doi: 10.1038/s41598-021-93591-0. Sci Rep. 2021. PMID: 34253772 Free PMC article.
-
Tissue-Level Cardiac Electrophysiology Studied in Murine Myocardium Using a Microelectrode Array: Autonomic and Thermal Modulation.J Membr Biol. 2017 Oct;250(5):471-481. doi: 10.1007/s00232-017-9973-y. Epub 2017 Aug 1. J Membr Biol. 2017. PMID: 28766006 Free PMC article.
-
The ric-8b protein (resistance to inhibitors of cholinesterase 8b) is key to preserving contractile function in the adult heart.J Biol Chem. 2024 Jul;300(7):107470. doi: 10.1016/j.jbc.2024.107470. Epub 2024 Jun 13. J Biol Chem. 2024. PMID: 38879012 Free PMC article.
References
-
- Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. - PubMed
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. - PubMed
-
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–233. - PubMed
-
- Bohm M, Gierschik P, Jakobs KH, Pieske B, Schnabel P, Ungerer M, Erdmann E. Increase of Gi alpha in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990;82:1249–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

