Molecular mechanisms in signal transduction at the membrane
- PMID: 20495561
- PMCID: PMC3703790
- DOI: 10.1038/nsmb.1844
Molecular mechanisms in signal transduction at the membrane
Abstract
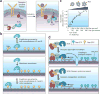
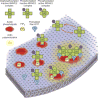
Signal transduction originates at the membrane, where the clustering of signaling proteins is a key step in transmitting a message. Membranes are difficult to study, and their influence on signaling is still only understood at the most rudimentary level. Recent advances in the biophysics of membranes, surveyed in this review, have highlighted a variety of phenomena that are likely to influence signaling activity, such as local composition heterogeneities and long-range mechanical effects. We discuss recent mechanistic insights into three signaling systems-Ras activation, Ephrin signaling and the control of actin nucleation-where the active role of membrane components is now appreciated and for which experimentation on the membrane is required for further understanding.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Activation of Ras requires the ERM-dependent link of actin to the plasma membrane.PLoS One. 2011;6(11):e27511. doi: 10.1371/journal.pone.0027511. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132106 Free PMC article.
-
Structures of actin-bound Wiskott-Aldrich syndrome protein homology 2 (WH2) domains of Spire and the implication for filament nucleation.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11757-62. doi: 10.1073/pnas.1005347107. Epub 2010 Jun 10. Proc Natl Acad Sci U S A. 2010. PMID: 20538977 Free PMC article.
-
Multivalent binding and facilitated diffusion account for the formation of the Grb2-Sos1 signaling complex in a cooperative manner.Biochemistry. 2012 Mar 13;51(10):2122-35. doi: 10.1021/bi3000534. Epub 2012 Mar 2. Biochemistry. 2012. PMID: 22360309 Free PMC article.
-
Bioblockades join the assault on small G protein signalling.Semin Cancer Biol. 2019 Feb;54:149-161. doi: 10.1016/j.semcancer.2018.01.001. Epub 2018 Jan 4. Semin Cancer Biol. 2019. PMID: 29307570 Review.
-
Biological Membrane Organization and Cellular Signaling.Chem Rev. 2019 May 8;119(9):5849-5880. doi: 10.1021/acs.chemrev.8b00439. Epub 2019 Feb 12. Chem Rev. 2019. PMID: 30747526 Review.
Cited by
-
Conformational Changes in the Epidermal Growth Factor Receptor: Role of the Transmembrane Domain Investigated by Coarse-Grained MetaDynamics Free Energy Calculations.J Am Chem Soc. 2016 Aug 24;138(33):10611-22. doi: 10.1021/jacs.6b05602. Epub 2016 Aug 11. J Am Chem Soc. 2016. PMID: 27459426 Free PMC article.
-
Monitoring shifts in the conformation equilibrium of the membrane protein cytochrome P450 reductase (POR) in nanodiscs.J Biol Chem. 2012 Oct 5;287(41):34596-603. doi: 10.1074/jbc.M112.400085. Epub 2012 Aug 13. J Biol Chem. 2012. PMID: 22891242 Free PMC article.
-
The Role of Signaling via Aqueous Pore Formation in Resistance Responses to Amphotericin B.Antimicrob Agents Chemother. 2016 Aug 22;60(9):5122-9. doi: 10.1128/AAC.00878-16. Print 2016 Sep. Antimicrob Agents Chemother. 2016. PMID: 27381391 Free PMC article. Review.
-
Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction.Mol Biol Cell. 2019 Nov 15;30(24):2996-3012. doi: 10.1091/mbc.E18-12-0823. Epub 2019 Oct 10. Mol Biol Cell. 2019. PMID: 31599693 Free PMC article.
-
Plant cell polarity as the nexus of tissue mechanics and morphogenesis.Nat Plants. 2021 Dec;7(12):1548-1559. doi: 10.1038/s41477-021-01021-w. Epub 2021 Dec 9. Nat Plants. 2021. PMID: 34887521 Review.
References
-
- Bhattacharyya RP, Remenyi A, Yeh BJ, Lim WA. Domains, motifs, and scaffolds: the role of modular interactions in the evolution and wiring of cell signaling circuits. Annu Rev Biochem. 2006;75:655–680. - PubMed
-
- Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. - PubMed
-
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

