Helicobacter pylori: gastric cancer and beyond
- PMID: 20495574
- PMCID: PMC2957472
- DOI: 10.1038/nrc2857
Helicobacter pylori: gastric cancer and beyond
Erratum in
- Nat Rev Cancer. 2010 Aug;10(8):593
Abstract
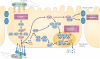
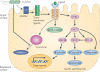
Helicobacter pylori is the dominant species of the human gastric microbiome, and colonization causes a persistent inflammatory response. H. pylori-induced gastritis is the strongest singular risk factor for cancers of the stomach; however, only a small proportion of infected individuals develop malignancy. Carcinogenic risk is modified by strain-specific bacterial components, host responses and/or specific host-microbe interactions. Delineation of bacterial and host mediators that augment gastric cancer risk has profound ramifications for both physicians and biomedical researchers as such findings will not only focus the prevention approaches that target H. pylori-infected human populations at increased risk for stomach cancer but will also provide mechanistic insights into inflammatory carcinomas that develop beyond the gastric niche.
Figures



References
-
- Peek RM, Jr., Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev. Cancer. 2002;2:28–37. - PubMed
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-- First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. - PubMed
-
- Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin. Microbiol Infect. 2009;15:971–976. - PubMed
-
- Wong BC, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. [One of the first large, randomized placebo-controlled trials to examine the effect of H. pylori eradication on the incidence of gastric cancer.] - PubMed
-
- Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med. 2007;167:821–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

