Primary cilia disappear in rat podocytes during glomerular development
- PMID: 20495826
- PMCID: PMC2898502
- DOI: 10.1007/s00441-010-0983-7
Primary cilia disappear in rat podocytes during glomerular development
Abstract
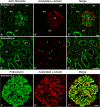
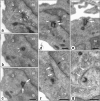
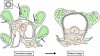
Most tubular epithelial cell types express primary cilia, and mutations of primary-cilium-associated proteins are well known to cause several kinds of cystic renal disease. However, until now, it has been unclear whether mammalian podocytes express primary cilia in vivo. In this study, we determined whether primary cilia are present in the podocytes of rat immature and mature glomeruli by means of transmission electron microscopy of serial ultrathin sections. In immature glomeruli of fetal rats, podocytes express the primary cilia with high percentages at the S-shaped body (88 +/- 5%, n = 3), capillary loop (95 +/- 4%, n = 4), and maturing glomerulus (76 +/- 13%, n = 5) stages. The percentage of ciliated podocytes was significantly lower at the maturing glomerulus stage than at the former two stages. In mature glomeruli of adult rats, ciliated podocytes were not found at all (0 +/- 0%, n = 11). These findings indicate that the primary cilia gradually disappear in rat podocytes during glomerular development. Since glomerular filtration rate increases during development, the primary cilia on the podocytes are subjected to a stronger bending force. Thus, the disappearance of the primary cilia presumably prevents the entry of excessive calcium-ions via the cilium-associated polycystin complexes and the disturbance of intracellular signaling cascades in mature podocytes.
Figures







Similar articles
-
Glomeruli from patients with nephrin mutations show increased number of ciliated and poorly differentiated podocytes.Acta Histochem. 2018 Nov;120(8):748-756. doi: 10.1016/j.acthis.2018.08.015. Epub 2018 Sep 5. Acta Histochem. 2018. PMID: 30193978
-
Podocyte number in the maturing rat kidney.Am J Nephrol. 2011;33(1):91-6. doi: 10.1159/000322701. Epub 2010 Dec 22. Am J Nephrol. 2011. PMID: 21196721 Free PMC article.
-
Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein.J Histochem Cytochem. 1991 Aug;39(8):1047-56. doi: 10.1177/39.8.1856454. J Histochem Cytochem. 1991. PMID: 1856454
-
Drebrin in Renal Glomeruli.Adv Exp Med Biol. 2017;1006:337-345. doi: 10.1007/978-4-431-56550-5_20. Adv Exp Med Biol. 2017. PMID: 28865030 Review.
-
Ontogenetic development of the filtration barrier.Nephron Exp Nephrol. 2007;106(2):e44-50. doi: 10.1159/000101792. Epub 2007 Jun 6. Nephron Exp Nephrol. 2007. PMID: 17570939 Review.
Cited by
-
Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy.Sci Rep. 2015 Mar 11;5:8993. doi: 10.1038/srep08993. Sci Rep. 2015. PMID: 25759085 Free PMC article.
-
The complexity of the cilium: spatiotemporal diversity of an ancient organelle.Curr Opin Cell Biol. 2018 Dec;55:139-149. doi: 10.1016/j.ceb.2018.08.001. Epub 2018 Aug 20. Curr Opin Cell Biol. 2018. PMID: 30138887 Free PMC article. Review.
-
Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis.Pediatr Nephrol. 2017 Mar;32(3):405-417. doi: 10.1007/s00467-016-3358-9. Epub 2016 Mar 23. Pediatr Nephrol. 2017. PMID: 27008645 Review.
-
Three-Dimensional Architecture of Glomerular Endothelial Cells Revealed by FIB-SEM Tomography.Front Cell Dev Biol. 2021 Mar 11;9:653472. doi: 10.3389/fcell.2021.653472. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777962 Free PMC article.
-
Rho GTPases in kidney physiology and diseases.Small GTPases. 2022 Jan;13(1):141-161. doi: 10.1080/21541248.2021.1932402. Epub 2021 Jun 17. Small GTPases. 2022. PMID: 34138686 Free PMC article.
References
-
- Andrews PM. Scanning electron microscopy of human and rhesus monkey kidneys. Lab Invest. 1975;32:510–518. - PubMed
-
- Braun EJ, Dantzler WH. Vertebrate renal system. In: Dantzler WH, editor. Handbook of physiology, section 13. Comparative physiology. 1. Oxford: Oxford University Press; 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

