Reactive oxygen species and small-conductance calcium-dependent potassium channels are key mediators of inflammation-induced hypotension and shock
- PMID: 20496172
- PMCID: PMC2921058
- DOI: 10.1007/s00109-010-0633-2
Reactive oxygen species and small-conductance calcium-dependent potassium channels are key mediators of inflammation-induced hypotension and shock
Abstract
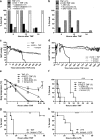
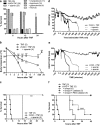
Septic shock is associated with life-threatening vasodilation and hypotension. To cause vasodilation, vascular endothelium may release nitric oxide (NO), prostacyclin (PGI2), and the elusive endothelium-derived hyperpolarizing factor (EDHF). Although NO is critical in controlling vascular tone, inhibiting NO in septic shock does not improve outcome, on the contrary, precipitating the search for alternative therapeutic targets. Using a hyperacute tumor necrosis factor (TNF)-induced shock model in mice, we found that shock can develop independently of the known vasodilators NO, cGMP, PGI2, or epoxyeicosatrienoic acids. However, the antioxidant tempol efficiently prevented hypotension, bradycardia, hypothermia, and mortality, indicating the decisive involvement of reactive oxygen species (ROS) in these phenomena. Also, in classical TNF or lipopolysaccharide-induced shock models, tempol protected significantly. Experiments with (cell-permeable) superoxide dismutase or catalase, N-acetylcysteine and apocynin suggest that the ROS-dependent shock depends on intracellular (*)OH radicals. Potassium channels activated by ATP (K(ATP)) or calcium (K(Ca)) are important mediators of vascular relaxation. While NO and PGI2-induced vasodilation involves K(ATP) and large-conductance BK(Ca) channels, small-conductance SK(Ca) channels mediate vasodilation induced by EDHF. Interestingly, also SK(Ca) inhibition completely prevented the ROS-dependent shock. Our data thus indicate that intracellular (*)OH and SK(Ca) channels represent interesting new therapeutic targets for inflammatory shock. Moreover, they may also explain why antioxidants other than tempol fail to provide survival benefit during shock.
Figures
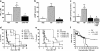

Similar articles
-
Role of EDHF in type 2 diabetes-induced endothelial dysfunction.Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H1982-8. doi: 10.1152/ajpheart.01261.2007. Epub 2008 Sep 12. Am J Physiol Heart Circ Physiol. 2008. PMID: 18790831 Free PMC article.
-
Critical role for small and large conductance calcium-dependent potassium channels in endotoxemia and TNF toxicity.Shock. 2008 May;29(5):577-82. doi: 10.1097/shk.0b013e31815071e9. Shock. 2008. PMID: 18467968
-
[Potassium channels in blood vessels: their role in health and disease].Postepy Hig Med Dosw (Online). 2007 Oct 17;61:596-605. Postepy Hig Med Dosw (Online). 2007. PMID: 17971762 Review. Polish.
-
Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels.Circulation. 2003 Feb 11;107(5):769-76. doi: 10.1161/01.cir.0000047278.28407.c2. Circulation. 2003. PMID: 12578883
-
Calcium-activated potassium channels - a therapeutic target for modulating nitric oxide in cardiovascular disease?Expert Opin Ther Targets. 2010 Aug;14(8):825-37. doi: 10.1517/14728222.2010.500616. Expert Opin Ther Targets. 2010. PMID: 20560781 Review.
Cited by
-
MAPK-activated protein kinase 2-deficiency causes hyperacute tumor necrosis factor-induced inflammatory shock.BMC Physiol. 2014 Sep 4;14:5. doi: 10.1186/s12899-014-0005-1. BMC Physiol. 2014. PMID: 25185746 Free PMC article.
-
Highly sensitive detection of lipopolysaccharides using an aptasensor based on hybridization chain reaction.Sci Rep. 2016 Jul 12;6:29524. doi: 10.1038/srep29524. Sci Rep. 2016. PMID: 27404735 Free PMC article.
-
Safe TNF-based antitumor therapy following p55TNFR reduction in intestinal epithelium.J Clin Invest. 2013 Jun;123(6):2590-603. doi: 10.1172/JCI65624. Epub 2013 May 15. J Clin Invest. 2013. PMID: 23676465 Free PMC article.
-
Salmonella and Reactive Oxygen Species: A Love-Hate Relationship.J Innate Immun. 2019;11(3):216-226. doi: 10.1159/000496370. Epub 2019 Apr 3. J Innate Immun. 2019. PMID: 30943492 Free PMC article. Review.
-
The soluble guanylate cyclase activator BAY 58-2667 protects against morbidity and mortality in endotoxic shock by recoupling organ systems.PLoS One. 2013 Aug 28;8(8):e72155. doi: 10.1371/journal.pone.0072155. eCollection 2013. PLoS One. 2013. PMID: 24015214 Free PMC article.
References
-
- Nguyen HB, Rivers EP, Abrahamian FM, Moran GJ, Abraham E, Trzeciak S, Huang DT, Osborn T, Stevens D, Talan DA. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48:28–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

