A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA
- PMID: 20496177
- PMCID: PMC3338170
- DOI: 10.1007/s00520-010-0911-0
A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a troublesome chronic symptom that has no proven pharmacologic treatment. The purpose of this double-blind randomized placebo-controlled trial was to evaluate a novel compounded topical gel for this problem.
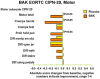
Methods: Patients with CIPN were randomized to baclofen 10 mg, amitriptyline HCL 40 mg, and ketamine 20 mg in a pluronic lecithin organogel (BAK-PLO) versus placebo (PLO) to determine its effect on numbness, tingling, pain, and function. The primary endpoint was the baseline-adjusted sensory subscale of the EORTC QLQ-CIPN20, at 4 weeks.
Results: Data in 208 patients reveal a trend for improvement that is greater in the BAK-PLO arm over placebo in both the sensory (p = 0.053) and motor subscales (p = 0.021). The greatest improvements were related to the symptoms of tingling, cramping, and shooting/burning pain in the hands as well as difficulty in holding a pen. There were no undesirable toxicities associated with the BAK-PLO and no evidence of systemic toxicity.
Conclusion: Topical treatment with BAK-PLO appears to somewhat improve symptoms of CIPN. This topical gel was well tolerated, without evident systemic toxicity. Further research is needed with increased doses to better clarify the clinical role of this treatment in CIPN.
Figures
References
-
- Cavaletti G, Bogliun G, Marzorati L, et al. Peripheral neurotoxicity of Taxol in patients previously treated with cisplatin. Cancer. 1995;75:1141–1150. - PubMed
-
- Cavaletti G, Fabbrica D, Minoia C, et al. Carboplatin toxic effects on the peripheral nervous system of the rat. Ann Oncol. 1998;9:443–447. - PubMed
-
- Ocean JA, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–625. - PubMed
-
- Pace A, Bove L, Aloe A, et al. Paclitaxel neurotoxicity: clinical andneurophysiological study of 23 patients. Ital JNeurol Sci. 1997;18:73–79. - PubMed
-
- Rowinsky EK, Chaudry V, Cornblath DR, et al. Neurotoxicity of taxol. J Natl Cancer Inst Monogr. 1993;15:107–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA035267/CA/NCI NIH HHS/United States
- CA124477/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- K05 CA124477/CA/NCI NIH HHS/United States
- CA-35195/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35448/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- R21 CA131795/CA/NCI NIH HHS/United States
- U10 CA035448/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- U10 CA035195/CA/NCI NIH HHS/United States
- CA-35103/CA/NCI NIH HHS/United States
- CA-63848/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- CA-35267/CA/NCI NIH HHS/United States
- CA-63849/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical