A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy
- PMID: 20496415
- PMCID: PMC3026777
- DOI: 10.1002/art.27572
A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy
Abstract
Objective: Myofiber necrosis without prominent inflammation is a nonspecific finding in patients with dystrophies and toxic or immune-mediated myopathies. However, the etiology of a necrotizing myopathy is often obscure, and the question of which patients would benefit from immunosuppression remains unanswered. The aim of this study was to identify novel autoantibodies in patients with necrotizing myopathy.
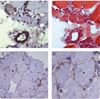
Methods: Muscle biopsy specimens and serum samples were available for 225 patients with myopathy. Antibody specificities were determined by performing immunoprecipitations from (35)S-methionine-labeled HeLa cell lysates. Selected biopsy specimens were stained for membrane attack complex, class I major histocompatibility complex (MHC), and endothelial cell marker CD31.
Results: Muscle biopsy specimens from 38 of 225 patients showed predominantly myofiber necrosis. Twelve of these patients had a known autoantibody association with or other etiology for their myopathy. Sixteen of the remaining 26 sera immunoprecipitated 200-kd and 100-kd proteins; this specificity was observed in only 1 of 187 patients without necrotizing myopathy. Patients with the anti-200/100 autoantibody specificity had proximal weakness (100%), high creatine kinase levels (mean maximum 10,333 IU/liter), and an irritable myopathy on electromyography (88%). Sixty-three percent of these patients had been exposed to statins prior to the onset of weakness. All patients responded to immunosuppressive therapy, and many experienced a relapse of weakness when the medication was tapered. Immunohistochemical studies showed membrane attack complex on small blood vessels in 6 of 8 patients and on the surface of non-necrotic myofibers in 4 of 8 patients. Five of 8 patients had abnormal capillary morphology, and 4 of 8 patients expressed class I MHC on the surface of non-necrotic myofibers.
Conclusion: An anti-200/100-kd specificity defines a subgroup of patients with necrotizing myopathy who previously were considered to be autoantibody negative. We propose that these patients have an immune-mediated myopathy that is frequently associated with prior statin use and should be treated with immunosuppressive therapy.
Figures



Comment in
-
"Muscles … and bones".Arthritis Rheum. 2010 Sep;62(9):2616-8. doi: 10.1002/art.27569. Arthritis Rheum. 2010. PMID: 20496418 No abstract available.
References
-
- Targoff IN. Autoantibodies and their significance in myositis. Curr Rheumatol Rep. 2008 Aug;10(4):333–340. - PubMed
-
- Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990 Sep;33(9):1361–1370. - PubMed
-
- Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 2004 Jan;50(1):209–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

