Recent development in PET instrumentation
- PMID: 20497121
- PMCID: PMC3697478
- DOI: 10.2174/138920110792246555
Recent development in PET instrumentation
Abstract
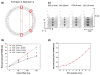
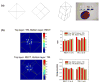
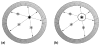
Positron emission tomography (PET) is used in the clinic and in vivo small animal research to study molecular processes associated with diseases such as cancer, heart disease, and neurological disorders, and to guide the discovery and development of new treatments. This paper reviews current challenges of advancing PET technology and some of newly developed PET detectors and systems. The paper focuses on four aspects of PET instrumentation: high photon detection sensitivity; improved spatial resolution; depth-of-interaction (DOI) resolution and time-of-flight (TOF). Improved system geometry, novel non-scintillator based detectors, and tapered scintillation crystal arrays are able to enhance the photon detection sensitivity of a PET system. Several challenges for achieving high resolution with standard scintillator-based PET detectors are discussed. Novel detectors with 3-D positioning capability have great potential to be deployed in PET for achieving spatial resolution better than 1 mm, such as cadmium-zinc-telluride (CZT) and position-sensitive avalanche photodiodes (PSAPDs). DOI capability enables a PET system to mitigate parallax error and achieve uniform spatial resolution across the field-of-view (FOV). Six common DOI designs, as well as advantages and limitations of each design, are discussed. The availability of fast scintillation crystals such as LaBr(3), and the silicon photomultiplier (SiPM) greatly advances TOF-PET development. Recent instrumentation and initial results of clinical trials are briefly presented. If successful, these technology advances, together with new probe molecules, will substantially enhance the molecular sensitivity of PET and thus increase its role in preclinical and clinical research as well as evaluating and managing disease in the clinic.
Figures








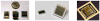
References
-
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. - PubMed
-
- Nestle U, Weber, Hentschel WM, Grosu AL. Biological imaging in radiation therapy: role of positron emission tomography. Phys Med Biol. 2009;54:R1–25. - PubMed
-
- Rohren EM, Turkington TG, Coleman E. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

