The sigma(E) stress response is required for stress-induced mutation and amplification in Escherichia coli
- PMID: 20497332
- PMCID: PMC2909356
- DOI: 10.1111/j.1365-2958.2010.07213.x
The sigma(E) stress response is required for stress-induced mutation and amplification in Escherichia coli
Abstract
Pathways of mutagenesis are induced in microbes under adverse conditions controlled by stress responses. Control of mutagenesis by stress responses may accelerate evolution specifically when cells are maladapted to their environments, i.e. are stressed. Stress-induced mutagenesis in the Escherichia coli Lac assay occurs either by 'point' mutation or gene amplification. Point mutagenesis is associated with DNA double-strand-break (DSB) repair and requires DinB error-prone DNA polymerase and the SOS DNA-damage- and RpoS general-stress responses. We report that the RpoE envelope-protein-stress response is also required. In a screen for mutagenesis-defective mutants, we isolated a transposon insertion in the rpoE P2 promoter. The insertion prevents rpoE induction during stress, but leaves constitutive expression intact, and allows cell viability. rpoE insertion and suppressed null mutants display reduced point mutagenesis and maintenance of amplified DNA. Furthermore, sigma(E) acts independently of stress responses previously implicated: SOS/DinB and RpoS, and of sigma(32), which was postulated to affect mutagenesis. I-SceI-induced DSBs alleviated much of the rpoE phenotype, implying that sigma(E) promoted DSB formation. Thus, a third stress response and stress input regulate DSB-repair-associated stress-induced mutagenesis. This provides the first report of mutagenesis promoted by sigma(E), and implies that extracytoplasmic stressors may affect genome integrity and, potentially, the ability to evolve.
Figures
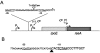
 ); SMR8844 rpoE::Tn[pTrc] (▴); SMR8846, rpoE+[pYYF] (•); SMR8845, rpoE+[pTrc] (♦). B. Growth curve following IPTG addition. Symbols as in (A).
); SMR8844 rpoE::Tn[pTrc] (▴); SMR8846, rpoE+[pYYF] (•); SMR8845, rpoE+[pTrc] (♦). B. Growth curve following IPTG addition. Symbols as in (A).
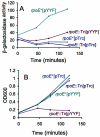
 ); rpoE2072::Tn10dCam, SMR5236 (♦). A. Representative experiment performed at 30°C. Values are means ± one standard error of the mean (SEM) for eight independent cultures of each strain. Where not visible, error bars are smaller than the symbol. A second experiment at 30°C gave similar results. B. Relative viability of the Lac- population monitored per Harris et al. (1996) beginning on the day after plating (day 1) for the experiment presented in (A). Values are means ± SEM for data from six selection plates. Because Lac+ mutant cells form colonies that are visible 2 days later (McKenzie et al., 1998), the day 3 Lac+ colony counts pertain to the day 1 viable cell measurements, and day 5 Lac+ colonies to the day 3 viable cells, etc. To make this comparison easier, we have shifted the viability data (B) 2 days rightward (the day 1 viability data are presented on day 3, etc.) for easier comparison with (A). C. Lac+ colony formation rates at 37° from multiple experiments. Lac+ colonies per day were calculated from colonies appearing from days 3–5 for seven independent stress-induced mutation assays and fold-difference between rates for SMR4562, rpoE+ and SMR5236, rpoE2072::Tn10dCam presented. Viability of all cultures was monitored per Harris et al. (1996). Mean ± SEM for the seven experiments is shown in last row of table. As observed previously, overall mutation rates are higher at 30°C than 37°C, although mutations that decrease mutagenesis do so similarly at both temperatures [Ponder et al. (2005) and A versus C].
); rpoE2072::Tn10dCam, SMR5236 (♦). A. Representative experiment performed at 30°C. Values are means ± one standard error of the mean (SEM) for eight independent cultures of each strain. Where not visible, error bars are smaller than the symbol. A second experiment at 30°C gave similar results. B. Relative viability of the Lac- population monitored per Harris et al. (1996) beginning on the day after plating (day 1) for the experiment presented in (A). Values are means ± SEM for data from six selection plates. Because Lac+ mutant cells form colonies that are visible 2 days later (McKenzie et al., 1998), the day 3 Lac+ colony counts pertain to the day 1 viable cell measurements, and day 5 Lac+ colonies to the day 3 viable cells, etc. To make this comparison easier, we have shifted the viability data (B) 2 days rightward (the day 1 viability data are presented on day 3, etc.) for easier comparison with (A). C. Lac+ colony formation rates at 37° from multiple experiments. Lac+ colonies per day were calculated from colonies appearing from days 3–5 for seven independent stress-induced mutation assays and fold-difference between rates for SMR4562, rpoE+ and SMR5236, rpoE2072::Tn10dCam presented. Viability of all cultures was monitored per Harris et al. (1996). Mean ± SEM for the seven experiments is shown in last row of table. As observed previously, overall mutation rates are higher at 30°C than 37°C, although mutations that decrease mutagenesis do so similarly at both temperatures [Ponder et al. (2005) and A versus C].

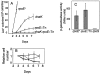
 ); SMR8862 dnaK (▴); SMR5236 rpoE::Tn (♦); SMR8863 rpoE::Tn dnaK (•). Assay was performed at 37°C as described in Experimental procedures. Values are means ± one SEM for six independent cultures of each strain in one experiment. Three experiments gave similar results. B. Viability of all cultures was monitored per Harris et al. (1996). Strains and symbols are as in (A) but with open symbols. C. Activity of the RpoS-dependent katE promoter is not diminished by rpoE2072::Tn10dCam. β-Galactosidase activity from a katE::lacZ fusion was measured in saturated LBH cultures in strains SL590, rpoE+; SMR8919, rpoE2072::Tn; and CH1761, rpoS::FRTKan. The means ± range of two experiments are shown. Error bars are too small to see for the rpoS strain.
); SMR8862 dnaK (▴); SMR5236 rpoE::Tn (♦); SMR8863 rpoE::Tn dnaK (•). Assay was performed at 37°C as described in Experimental procedures. Values are means ± one SEM for six independent cultures of each strain in one experiment. Three experiments gave similar results. B. Viability of all cultures was monitored per Harris et al. (1996). Strains and symbols are as in (A) but with open symbols. C. Activity of the RpoS-dependent katE promoter is not diminished by rpoE2072::Tn10dCam. β-Galactosidase activity from a katE::lacZ fusion was measured in saturated LBH cultures in strains SL590, rpoE+; SMR8919, rpoE2072::Tn; and CH1761, rpoS::FRTKan. The means ± range of two experiments are shown. Error bars are too small to see for the rpoS strain.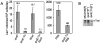
Similar articles
-
Persistent damaged bases in DNA allow mutagenic break repair in Escherichia coli.PLoS Genet. 2017 Jul 20;13(7):e1006733. doi: 10.1371/journal.pgen.1006733. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28727736 Free PMC article.
-
DinB upregulation is the sole role of the SOS response in stress-induced mutagenesis in Escherichia coli.Genetics. 2009 May;182(1):55-68. doi: 10.1534/genetics.109.100735. Epub 2009 Mar 6. Genetics. 2009. PMID: 19270270 Free PMC article.
-
The Small RNA GcvB Promotes Mutagenic Break Repair by Opposing the Membrane Stress Response.J Bacteriol. 2016 Nov 18;198(24):3296-3308. doi: 10.1128/JB.00555-16. Print 2016 Dec 15. J Bacteriol. 2016. PMID: 27698081 Free PMC article.
-
Adaptive mutation and amplification in Escherichia coli: two pathways of genome adaptation under stress.Res Microbiol. 2004 Jun;155(5):352-9. doi: 10.1016/j.resmic.2004.01.020. Res Microbiol. 2004. PMID: 15207867 Review.
-
Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli.Res Microbiol. 2009 Nov;160(9):667-76. doi: 10.1016/j.resmic.2009.08.014. Epub 2009 Sep 16. Res Microbiol. 2009. PMID: 19765651 Review.
Cited by
-
"Metabolic burden" explained: stress symptoms and its related responses induced by (over)expression of (heterologous) proteins in Escherichia coli.Microb Cell Fact. 2024 Mar 30;23(1):96. doi: 10.1186/s12934-024-02370-9. Microb Cell Fact. 2024. PMID: 38555441 Free PMC article. Review.
-
Identity and function of a large gene network underlying mutagenic repair of DNA breaks.Science. 2012 Dec 7;338(6112):1344-8. doi: 10.1126/science.1226683. Science. 2012. PMID: 23224554 Free PMC article.
-
Oxygen and RNA in stress-induced mutation.Curr Genet. 2018 Aug;64(4):769-776. doi: 10.1007/s00294-017-0801-9. Epub 2018 Jan 2. Curr Genet. 2018. PMID: 29294174 Free PMC article. Review.
-
Stress-induced mutagenesis and complex adaptation.Proc Biol Sci. 2014 Oct 7;281(1792):20141025. doi: 10.1098/rspb.2014.1025. Proc Biol Sci. 2014. PMID: 25143032 Free PMC article.
-
The Effects of Natural Products and Environmental Conditions on Antimicrobial Resistance.Molecules. 2021 Jul 14;26(14):4277. doi: 10.3390/molecules26144277. Molecules. 2021. PMID: 34299552 Free PMC article. Review.
References
-
- Ades S. Regulation by destruction: design of the sigmaE envelope stress response. Curr Opin Microbiol. 2008;11:535–540. - PubMed
-
- Ades SE. Control of the alternative sigma factor sigmaE in Escherichia coli. Curr Opin Microbiol. 2004;7:157–162. - PubMed
-
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. - PubMed
-
- Alba BM, Zhong HJ, Pelayo JC, Gross CA. degS (hhoB) is an essential Escherichia coli gene whose indispensable function is to provide sigma (E) activity. Mol Microbiol. 2001;40:1323–1333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

