Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs
- PMID: 20497536
- PMCID: PMC2881938
- DOI: 10.1186/1475-2875-9-136
Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs
Abstract
Background: There is renewed acknowledgement that targeting gametocytes is essential for malaria control and elimination efforts. Simple mathematical models were fitted to data from clinical trials in order to determine the mean gametocyte circulation time and duration of gametocyte carriage in treated malaria patients.
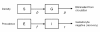
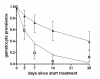
Methods: Data were used from clinical trials from East Africa. The first trial compared non-artemisinin combination therapy (non-ACT: sulphadoxine-pyrimethamine (SP) plus amodiaquine) and artemisinin-based combination therapy (ACT: SP plus artesunate (AS) or artemether-lumefantrine). The second trial compared ACT (SP+AS) with ACT in combination with a single dose of primaquine (ACT-PQ: SP+AS+PQ). Mature gametocytes were quantified in peripheral blood samples by nucleic acid sequence based amplification. A simple deterministic compartmental model was fitted to gametocyte densities to estimate the circulation time per gametocyte; a similar model was fitted to gametocyte prevalences to estimate the duration of gametocyte carriage after efficacious treatment.
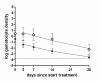
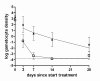
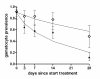
Results: The mean circulation time of gametocytes was 4.6-6.5 days. After non-ACT treatment, patients were estimated to carry gametocytes for an average of 55 days (95% CI 28.7 - 107.7). ACT reduced the duration of gametocyte carriage fourfold to 13.4 days (95% CI 10.2-17.5). Addition of PQ to ACT resulted in a further fourfold reduction of the duration of gametocyte carriage.
Conclusions: These findings confirm previous estimates of the circulation time of gametocytes, but indicate a much longer duration of (low density) gametocyte carriage after apparently successful clearance of asexual parasites. ACT shortened the period of gametocyte carriage considerably, and had the most pronounced effect on mature gametocytes when combined with PQ.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

