Modeling the interaction of binary and ternary mixtures of estradiol with bisphenol A and bisphenol AF in an in vitro estrogen-mediated transcriptional activation assay (T47D-KBluc)
- PMID: 20498000
- PMCID: PMC2905410
- DOI: 10.1093/toxsci/kfq156
Modeling the interaction of binary and ternary mixtures of estradiol with bisphenol A and bisphenol AF in an in vitro estrogen-mediated transcriptional activation assay (T47D-KBluc)
Abstract
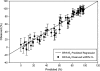
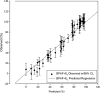
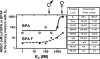
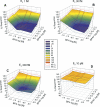
Exposure to xenoestrogens occurs against a backdrop to physiological levels of endogenous estrogens. Endogenous estrogen levels vary from low levels in early childhood to high levels during pregnancy and in young women. However, few studies have addressed how xenoestrogens interact with endogenous estrogens. The current study was designed to characterize the individual dose-response curves of estradiol-17beta (E(2)), bisphenol A (BPA), tetrabromo-bisphenol A (TBBPA), and bisphenol AF (BPAF, 4,4'-hexafluoroisopropylidene diphenol) on estrogen-dependent luciferase expression in T47D-KBluc cells and to determine how binary (8 x 8 factorial) and ternary (4 x 4 x 4 factorial) mixtures of an endogenous estrogen (E(2)) interact with BPA and/or BPAF. Log EC(50) and hillslope values with SEs, respectively, for individual compounds were as follows: E(2), -12.10M +/- 0.06071, 0.7702 +/- 0.1739; BPA, -6.679M +/- 0.08505, 1.194 +/- 0.2137; and BPAF, -7.648M +/- 0.05527, 1.273 +/- 0.1739. TBBPA was not evaluated in mixture studies because of its minimally estrogenic response at 3 x10(-5)M and elicited cytotoxicity at higher concentrations. Both the binary mixtures of E(2) with BPA and BPAF and the ternary mixture of E(2), BPA, and BPAF behaved in an additive manner. For binary mixtures, as E(2) concentration increased, higher concentrations of BPA and BPAF were necessary to induce a significant increase in the estrogenic response. Understanding the behavior of mixture interactions of xenoestrogens, like BPA and BPAF, with endogenous estrogens will allow a better assessment of the potential risk associated with exposure to these chemicals, individually or as mixtures.
Figures
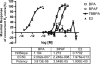
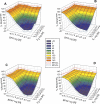
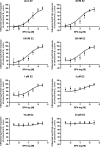
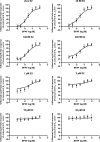
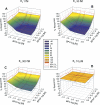
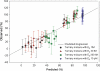
References
-
- Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol. In Vitro. 2008;22:225–231. - PubMed
-
- Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson AM. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Hum. Reprod. Update. 2006;12:341–349. - PubMed
-
- Bay K, Andersson AM, Skakkebaek NE. Estradiol levels in prepubertal boys and girls—analytical challenges. Int. J. Androl. 2004;27:266–273. - PubMed
-
- Bhavsar SP, Reiner EJ, Hayton A, Fletcher R, MacPherson K. Converting toxic equivalents (TEQ) of dioxins and dioxin-like compounds in fish from one toxic equivalency factor (TEF) scheme to another. Environ. Int. 2008;34:915–921. - PubMed
-
- Center for the Evaluation of Risks to Human Reproduction (CERHR) NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. Research Triangle Park, NC: NIEHS; 2008. - PubMed

