Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection
- PMID: 20498003
- PMCID: PMC2905408
- DOI: 10.1093/toxsci/kfq153
Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection
Abstract
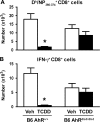
It has long been known that activation of the aryl hydrocarbon receptor (AhR) by ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses T cell-dependent immune responses; however, the underlying cellular targets and mechanism remain unclear. We have previously shown that AhR activation by TCDD reduces the proliferation and differentiation of influenza virus-specific CD8(+) T cells through an indirect mechanism; suggesting that accessory cells are critical AhR targets during infection. Respiratory dendritic cells (DCs) capture antigen, migrate to lymph nodes, and play a key role in activating naive CD8(+) T cells during respiratory virus infection. Herein, we report an examination of how AhR activation alters DCs in the lung and affects their trafficking to and function in the mediastinal lymph nodes (MLN) during infection with influenza virus. We show that AhR activation impairs lung DC migration and reduces the ability of DCs isolated from the MLN to activate naive CD8(+) T cells. Using novel AhR mutant mice, in which the AhR protein lacks its DNA-binding domain, we show that the suppressive effects of TCDD require that the activated AhR complex binds to DNA. These new findings suggest that AhR activation by chemicals from our environment impacts DC function to stimulate naive CD8(+) T cells and that immunoregulatory genes within DCs are critical targets of AhR. Moreover, our results reinforce the idea that environmental signals and AhR ligands may contribute to differential susceptibilities and responses to respiratory infection.
Figures

Similar articles
-
Environmental cues received during development shape dendritic cell responses later in life.PLoS One. 2018 Nov 9;13(11):e0207007. doi: 10.1371/journal.pone.0207007. eCollection 2018. PLoS One. 2018. PMID: 30412605 Free PMC article.
-
New insights into the role of the aryl hydrocarbon receptor in the function of CD11c⁺ cells during respiratory viral infection.Eur J Immunol. 2014 Jun;44(6):1685-1698. doi: 10.1002/eji.201343980. Epub 2014 Mar 19. Eur J Immunol. 2014. PMID: 24519489 Free PMC article.
-
Genome-Wide Transcriptional Analysis Reveals Novel AhR Targets That Regulate Dendritic Cell Function during Influenza A Virus Infection.Immunohorizons. 2019 Jun 17;3(6):219-235. doi: 10.4049/immunohorizons.1900004. Immunohorizons. 2019. PMID: 31356168 Free PMC article.
-
Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung.J Immunol. 2006 Nov 1;177(9):5819-28. doi: 10.4049/jimmunol.177.9.5819. J Immunol. 2006. PMID: 17056506
-
The aryl hydrocarbon receptor is a modulator of anti-viral immunity.Biochem Pharmacol. 2009 Feb 15;77(4):642-53. doi: 10.1016/j.bcp.2008.10.031. Epub 2008 Nov 5. Biochem Pharmacol. 2009. PMID: 19027719 Free PMC article. Review.
Cited by
-
You AhR what you eat?Nat Immunol. 2012 Jan 19;13(2):117-9. doi: 10.1038/ni.2213. Nat Immunol. 2012. PMID: 22261961 No abstract available.
-
Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo.PLoS One. 2013 Nov 14;8(11):e79819. doi: 10.1371/journal.pone.0079819. eCollection 2013. PLoS One. 2013. PMID: 24244565 Free PMC article.
-
Aryl Hydrocarbon Receptor-Signaling Regulates Early Leishmania major-Induced Cytokine Expression.Front Immunol. 2019 Oct 15;10:2442. doi: 10.3389/fimmu.2019.02442. eCollection 2019. Front Immunol. 2019. PMID: 31749794 Free PMC article.
-
Environmental cues received during development shape dendritic cell responses later in life.PLoS One. 2018 Nov 9;13(11):e0207007. doi: 10.1371/journal.pone.0207007. eCollection 2018. PLoS One. 2018. PMID: 30412605 Free PMC article.
-
Autoimmunity and infection in Sjögren's syndrome.Curr Opin Rheumatol. 2013 Jul;25(4):480-7. doi: 10.1097/BOR.0b013e32836200d2. Curr Opin Rheumatol. 2013. PMID: 23719365 Free PMC article. Review.
References
-
- Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat. Immunol. 2007;8:1060–1066. - PubMed
-
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8670–8675. - PMC - PubMed
-
- Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 2001;166:4627–4633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

