Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry
- PMID: 20498047
- PMCID: PMC2890849
- DOI: 10.1073/pnas.1002128107
Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry
Abstract
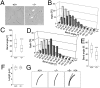
Airway epithelial cilia protect the mammalian respiratory system from harmful inhaled materials by providing the force necessary for effective mucociliary clearance. Ciliary beating is asymmetric, composed of clearly distinguished effective and recovery strokes. Neither the importance of nor the essential components responsible for the beating asymmetry has been directly elucidated. We report here that the beating asymmetry is crucial for ciliary function and requires tubulin glutamylation, a unique posttranslational modification that is highly abundant in cilia. WT murine tracheal cilia have an axoneme-intrinsic structural curvature that points in the direction of effective strokes. The axonemal curvature was lost in tracheal cilia from mice with knockout of a tubulin glutamylation-performing enzyme, tubulin tyrosine ligase-like protein 1. Along with the loss of axonemal curvature, the axonemes and tracheal epithelial cilia from these knockout (KO) mice lost beating asymmetry. The loss of beating asymmetry resulted in a reduction of cilia-generated fluid flow in trachea from the KO mice. The KO mice displayed a significant accumulation of mucus in the nasal cavity, and also emitted frequent coughing- or sneezing-like noises. Thus, the beating asymmetry is important for airway ciliary function. Our findings provide evidence that tubulin glutamylation is essential for ciliary function through the regulation of beating asymmetry, and provides insight into the molecular basis underlying the beating asymmetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


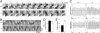

References
-
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. - PubMed
-
- Ferkol T, Leigh M. Primary ciliary dyskinesia and newborn respiratory distress. Semin Perinatol. 2006;30:335–340. - PubMed
-
- Morillas HN, Zariwala M, Knowles MR. Genetic causes of bronchiectasis: Primary ciliary dyskinesia. Respiration. 2007;74:252–263. - PubMed
-
- Nicastro D, et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

