NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes
- PMID: 20498052
- PMCID: PMC2890838
- DOI: 10.1073/pnas.0914867107
NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes
Abstract
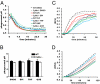
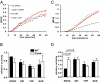
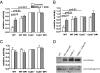
The phagosomal lumen in macrophages is the site of numerous interacting chemistries that mediate microbial killing, macromolecular degradation, and antigen processing. Using a non-hypothesis-based screen to explore the interconnectivity of phagosomal functions, we found that NADPH oxidase (NOX2) negatively regulates levels of proteolysis within the maturing phagosome of macrophages. Unlike the NOX2 mechanism of proteolytic control reported in dendritic cells, this phenomenon in macrophages is independent of changes to lumenal pH and is also independent of hydrolase delivery to the phagosome. We found that NOX2 mediates the inhibition of phagosomal proteolysis in macrophages through reversible oxidative inactivation of local cysteine cathepsins. We also show that NOX2 activity significantly compromises the phagosome's ability to reduce disulfides. These findings indicate that NOX2 oxidatively inactivates cysteine cathepsins through sustained ablation of the reductive capacity of the phagosomal lumen. This constitutes a unique mechanism of spatiotemporal control of phagosomal chemistries through the modulation of the local redox environment. In addition, this work further implicates the microbicidal effector NOX2 as a global modulator of phagosomal physiologies, particularly of those pertinent to antigen processing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
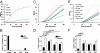
Similar articles
-
Ligation of FcγR Alters Phagosomal Processing of Protein via Augmentation of NADPH Oxidase Activity.Traffic. 2016 Jul;17(7):786-802. doi: 10.1111/tra.12396. Epub 2016 May 13. Traffic. 2016. PMID: 27020146
-
Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner.EMBO J. 2012 Feb 15;31(4):932-44. doi: 10.1038/emboj.2011.440. Epub 2011 Dec 13. EMBO J. 2012. PMID: 22157818 Free PMC article.
-
Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms.Blood. 2011 Oct 13;118(15):4199-208. doi: 10.1182/blood-2011-01-328906. Epub 2011 Aug 16. Blood. 2011. PMID: 21846901
-
Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes?Mol Cell Endocrinol. 2012 Apr 28;353(1-2):82-7. doi: 10.1016/j.mce.2011.10.005. Epub 2011 Oct 26. Mol Cell Endocrinol. 2012. PMID: 22056415 Review.
-
The phagosome and redox control of antigen processing.Free Radic Biol Med. 2018 Sep;125:53-61. doi: 10.1016/j.freeradbiomed.2018.03.040. Epub 2018 Mar 22. Free Radic Biol Med. 2018. PMID: 29578071 Review.
Cited by
-
Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism.Front Immunol. 2021 Feb 25;12:635021. doi: 10.3389/fimmu.2021.635021. eCollection 2021. Front Immunol. 2021. PMID: 33717180 Free PMC article. Review.
-
Gut redox and microbiome: charting the roadmap to T-cell regulation.Front Immunol. 2024 Aug 21;15:1387903. doi: 10.3389/fimmu.2024.1387903. eCollection 2024. Front Immunol. 2024. PMID: 39234241 Free PMC article. Review.
-
The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans.Biomolecules. 2015 May 4;5(2):702-23. doi: 10.3390/biom5020702. Biomolecules. 2015. PMID: 25946076 Free PMC article. Review.
-
Redundancy between Cysteine Cathepsins in Murine Experimental Autoimmune Encephalomyelitis.PLoS One. 2015 Jun 15;10(6):e0128945. doi: 10.1371/journal.pone.0128945. eCollection 2015. PLoS One. 2015. PMID: 26075905 Free PMC article.
-
NOX2 As a Target for Drug Development: Indications, Possible Complications, and Progress.Antioxid Redox Signal. 2015 Aug 10;23(5):375-405. doi: 10.1089/ars.2014.5862. Epub 2014 Mar 24. Antioxid Redox Signal. 2015. PMID: 24512192 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

