B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression
- PMID: 20498063
- PMCID: PMC2890809
- DOI: 10.1073/pnas.1004934107
B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression
Abstract
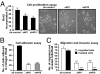
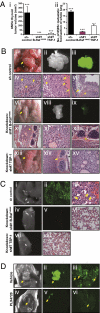
Although B-Raf(V600E) is the most common somatic mutation in papillary thyroid carcinoma (PTC), how it induces tumor aggressiveness is not fully understood. Using gene set enrichment analysis and in vitro and in vivo functional studies, we identified and validated a B-Raf(V600E) gene set signature associated with tumor progression in PTCs. An independent cohort of B-Raf(V600E)-positive PTCs showed significantly higher expression levels of many extracellular matrix genes compared with controls. We performed extensive in vitro and in vivo validations on thrombospondin-1 (TSP-1), because it has been previously shown to be important in the regulation of tumor angiogenesis and metastasis and is present in abundance in tumor stroma. Knockdown of B-Raf(V600E) resulted in TSP-1 down-regulation and a reduction of adhesion and migration/invasion of human thyroid cancer cells. Knockdown of TSP-1 resulted in a similar phenotype. B-Raf(V600E) cells in which either B-Raf(V600E) or TSP-1 were knocked down were implanted orthotopically into the thyroids of immunocompromised mice, resulting in significant reduction in tumor size and fewer pulmonary metastases from the primary carcinoma as compared with the control cells. Treatment of orthotopic thyroid tumors, initiated 1 week after tumor cell implantation with PLX4720, an orally available selective inhibitor of B-Raf(V600E), caused a significant tumor growth delay and decreased distant metastases, without evidence of toxicity. In conclusion, B-Raf(V600E) plays an important role in PTC progression through genes (i.e., TSP-1) important in tumor invasion and metastasis. Testing of a patient's thyroid cancer for B-Raf(V600E) will yield important information about potential tumor aggressiveness and also allow for future use of targeted therapies with selective B-Raf(V600E) inhibitors, such as PLX4720.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. 2007. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed September 16, 2009.
-
- Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–156. - PubMed
-
- Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. - PubMed
-
- Frasca F, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

