Control of bacterial iron homeostasis by manganese
- PMID: 20498065
- PMCID: PMC2890801
- DOI: 10.1073/pnas.1002342107
Control of bacterial iron homeostasis by manganese
Abstract
Perception and response to nutritional iron availability by bacteria are essential to control cellular iron homeostasis. The Irr protein from Bradyrhizobium japonicum senses iron through the status of heme biosynthesis to globally regulate iron-dependent gene expression. Heme binds directly to Irr to trigger its degradation. Here, we show that severe manganese limitation created by growth of a Mn(2+) transport mutant in manganese-limited media resulted in a cellular iron deficiency. In wild-type cells, Irr levels were attenuated under manganese limitation, resulting in reduced promoter occupancy of target genes and altered iron-dependent gene expression. Irr levels were high regardless of manganese availability in a heme-deficient mutant, indicating that manganese normally affects heme-dependent degradation of Irr. Manganese altered the secondary structure of Irr in vitro and inhibited binding of heme to the protein. We propose that manganese limitation destabilizes Irr under low-iron conditions by lowering the threshold of heme that can trigger Irr degradation. The findings implicate a mechanism for the control of iron homeostasis by manganese in a bacterium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




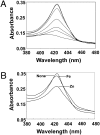
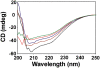

References
-
- Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. - PubMed
-
- Makui H, et al. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. - PubMed
-
- Garrick MD, et al. DMT1: A mammalian transporter for multiple metals. Biometals. 2003;16:41–54. - PubMed
-
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

