Receptor regulation of osmolyte homeostasis in neural cells
- PMID: 20498228
- PMCID: PMC2988502
- DOI: 10.1113/jphysiol.2010.190777
Receptor regulation of osmolyte homeostasis in neural cells
Abstract
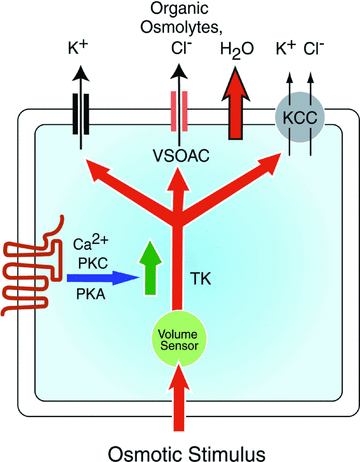
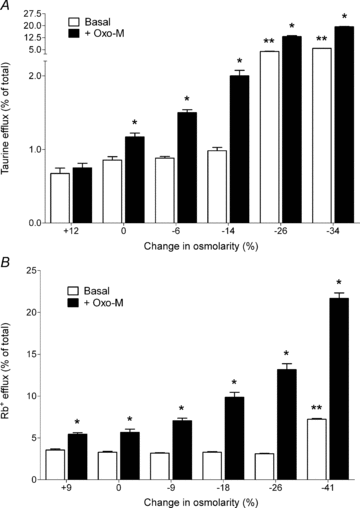
The capacity of cells to correct their volume in response to hyposmotic stress via the efflux of inorganic and organic osmolytes is well documented. However, the ability of cell-surface receptors, in particular G-protein-coupled receptors (GPCRs), to regulate this homeostatic mechanism has received much less attention. Mechanisms that underlie the regulation of cell volume are of particular importance to cells in the central nervous system because of the physical restrictions of the skull and the adverse impact that even small increases in cell volume can have on their function. Increases in brain volume are seen in hyponatraemia, which can arise from a variety of aetiologies and is the most frequently diagnosed electrolyte disorder in clinical practice. In this review we summarize recent evidence that the activation of GPCRs facilitates the volume-dependent efflux of osmolytes from neural cells and permits them to more efficiently respond to small, physiologically relevant, reductions in osmolarity. The characteristics of receptor-regulated osmolyte efflux, the signalling pathways involved and the physiological significance of receptor activation are discussed. In addition, we propose that GPCRs may also regulate the re-uptake of osmolytes into neural cells, but that the influx of organic and inorganic osmolytes is differentially regulated. The ability of neural cells to closely regulate osmolyte homeostasis through receptor-mediated alterations in both efflux and influx mechanisms may explain, in part at least, why the brain selectively retains its complement of inorganic osmolytes during chronic hyponatraemia, whereas its organic osmolytes are depleted.
Figures

Similar articles
-
Volume-dependent osmolyte efflux from neural tissues: regulation by G-protein-coupled receptors.J Neurochem. 2008 Sep;106(5):1998-2014. doi: 10.1111/j.1471-4159.2008.05510.x. Epub 2008 Jun 2. J Neurochem. 2008. PMID: 18518929 Free PMC article. Review.
-
Autocrine signaling involved in cell volume regulation: the role of released transmitters and plasma membrane receptors.J Cell Physiol. 2008 Jul;216(1):14-28. doi: 10.1002/jcp.21406. J Cell Physiol. 2008. PMID: 18300263 Review.
-
Signaling events during swelling and regulatory volume decrease.Neurochem Res. 2000 Oct;25(9-10):1301-14. doi: 10.1023/a:1007652330703. Neurochem Res. 2000. PMID: 11059803 Review.
-
The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis.J Invest Dermatol. 2004 Sep;123(3):516-21. doi: 10.1111/j.0022-202X.2004.23313.x. J Invest Dermatol. 2004. PMID: 15304091
-
Purinergic activation of anion conductance and osmolyte efflux in cultured rat hippocampal neurons.Am J Physiol Cell Physiol. 2008 Dec;295(6):C1550-60. doi: 10.1152/ajpcell.90605.2007. Epub 2008 Oct 15. Am J Physiol Cell Physiol. 2008. PMID: 18923056 Free PMC article.
Cited by
-
Osmotic Demyelination: From an Oligodendrocyte to an Astrocyte Perspective.Int J Mol Sci. 2019 Mar 5;20(5):1124. doi: 10.3390/ijms20051124. Int J Mol Sci. 2019. PMID: 30841618 Free PMC article. Review.
-
Hyponatremia Demystified: Integrating Physiology to Shape Clinical Practice.Adv Kidney Dis Health. 2023 Mar;30(2):85-101. doi: 10.1053/j.akdh.2022.11.004. Epub 2022 Dec 14. Adv Kidney Dis Health. 2023. PMID: 36868737 Free PMC article. Review.
-
Thrombin-facilitated efflux of D-[3H]-aspartate from cultured astrocytes and neurons under hyponatremia and chemical ischemia.Neurochem Res. 2014 Jul;39(7):1219-31. doi: 10.1007/s11064-014-1300-8. Epub 2014 Apr 5. Neurochem Res. 2014. PMID: 24706094
-
Fluid type influences acute hydration and muscle performance recovery in human subjects.J Int Soc Sports Nutr. 2019 Apr 4;16(1):15. doi: 10.1186/s12970-019-0282-y. J Int Soc Sports Nutr. 2019. PMID: 30947727 Free PMC article. Clinical Trial.
-
Choline Ameliorates Disease Phenotypes in Human iPSC Models of Rett Syndrome.Neuromolecular Med. 2016 Sep;18(3):364-77. doi: 10.1007/s12017-016-8421-y. Epub 2016 Jul 5. Neuromolecular Med. 2016. PMID: 27379379
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

