The membrane protein MiRP3 regulates Kv4.2 channels in a KChIP-dependent manner
- PMID: 20498229
- PMCID: PMC2916995
- DOI: 10.1113/jphysiol.2010.191395
The membrane protein MiRP3 regulates Kv4.2 channels in a KChIP-dependent manner
Abstract
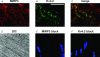
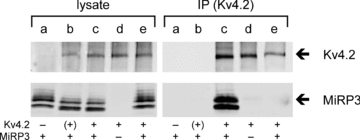
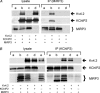
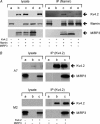
MiRP3, the single-span membrane protein encoded by KCNE4, is localized by immunofluorescence microscopy to the transverse tubules of murine cardiac myocytes. MiRP3 is found to co-localize with Kv4.2 subunits that contribute to cardiac transient outward potassium currents (I(to)). Whole-cell, voltage-clamp recordings of human MiRP3 and Kv4.2 expressed in a clonal cell line (tsA201) reveal MiRP3 to modulate Kv4.2 current activation, inactivation and recovery from inactivation. MiRP3 shifts the half-maximal voltage for activation (V(1/2)) approximately 20 mV and slows time to peak approximately 100%. In addition, MiRP3 slows inactivation approximately 100%, speeds recovery from inactivation approximately 30%, and enhances restored currents so they 'overshoot' baseline levels. The cytoplasmic accessory subunit KChIP2 also assembles with Kv4.2 in tsA201 cells to increase peak current, shift V(1/2) approximately 5 mV, slow time to peak approximately 10%, slow inactivation approximately 100%, and speed recovery from inactivation approximately 250% without overshoot. Simultaneous expression of all three subunits yields a biophysical profile unlike either accessory subunit alone, abolishes MiRP3-induced overshoot, and allows biochemical isolation of the ternary complex. Thus, regional heterogeneity in cardiac expression of MiRP3, Kv4.2 and KChIP2 in health and disease may establish the local attributes and magnitude of cardiac I(to).
Figures


References
-
- Abbott GW, Goldstein SA. A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs) Q Rev Biophys. 1998;31:357–398. - PubMed
-
- Abbott GW, Goldstein SA. Disease-associated mutations in KCNE potassium channel subunits (MiRPs) reveal promiscuous disruption of multiple currents and conservation of mechanism. FASEB J. 2002;16:390–400. - PubMed
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM051851/GM/NIGMS NIH HHS/United States
- K08 DK068188/DK/NIDDK NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 HL061657/HL/NHLBI NIH HHS/United States
- R01-HL071115/HL/NHLBI NIH HHS/United States
- RC1 HL099462/HL/NHLBI NIH HHS/United States
- R01 HL071115/HL/NHLBI NIH HHS/United States
- R01 HL105949/HL/NHLBI NIH HHS/United States
- R01GM51851/GM/NIGMS NIH HHS/United States
- DK068188/DK/NIDDK NIH HHS/United States
- R01 HL113003/HL/NHLBI NIH HHS/United States
- R01 NS058505/NS/NINDS NIH HHS/United States
- 1RC1HL099462-01/HL/NHLBI NIH HHS/United States

