Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons
- PMID: 20498231
- PMCID: PMC2916990
- DOI: 10.1113/jphysiol.2010.190066
Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons
Abstract
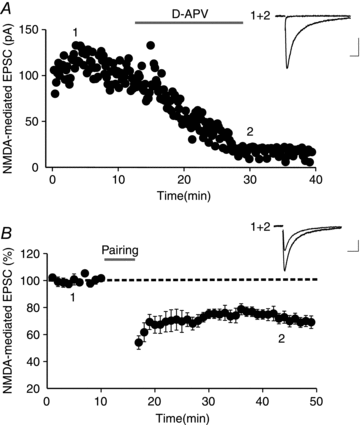
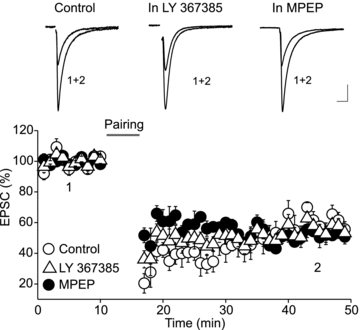
Endocannabinoids (eCBs) are lipid signalling molecules which play a key role in the regulation of synaptic transmission and plasticity in the central nervous system. Previous studies have reported that eCBs are released 'on demand' in the ventral tegmental area (VTA), a brain region critical for reward learning. However, their role in modulating the long-term plasticity of glutamate synapses of VTA dopamine (DA) neurons remains unknown. In the present study, we showed that low frequency afferent stimulation paired with moderate postsynaptic depolarization elicited an N-methyl-d-aspartate (NMDA) receptor-independent long-term depression (LTD) at glutamate synapses of VTA DA neurons. This form of LTD was caused by a decrease in the probability of glutamate release. Examination of the mechanisms underlying this form of LTD revealed that it was mediated by retrograde eCB signalling. In addition, we found that inhibition of 2-arachidonoyl glycerol biosynthesis blocked LTD induction, suggesting that 2-arachidonoyl glycerol is the most likely retrograde eCB messenger mediating LTD. The eCB-LTD induced at glutamate synapses of VTA DA neurons also required the inhibition of the presynaptic cAMP/PKA pathway. Taken together, these results reveal a critical role of eCBs in controlling the long-term plasticity of glutamate synapses in VTA DA neurons.
Figures




References
-
- Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Diherty P. Cloning of the first sn1-DGLs points to the spatial and temporal regulation of endocannabinoid signalling in the brain. J Cell Biol. 2003;163:463–468. - PMC - PubMed
-
- Chapin EM, Andrade R. A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current Ih. J Pharmacol Exp Ther. 2001;297:403–409. - PubMed
-
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

