Peyer's patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination
- PMID: 20498259
- PMCID: PMC2916292
- DOI: 10.1128/IAI.01411-09
Peyer's patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination
Abstract
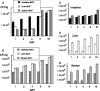
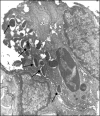
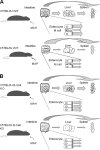
Mycobacterium avium subsp. paratuberculosis, the agent of Johne's disease, infects ruminant hosts by translocation through the intestinal mucosa. A number of studies have suggested that M. avium subsp. paratuberculosis interacts with M cells in the Peyer's patches of the small intestine. The invasion of the intestinal mucosa by M. avium subsp. paratuberculosis and Mycobacterium avium subsp. hominissuis, a pathogen known to interact with intestinal cells, was compared. M. avium subsp. paratuberculosis was capable of invading the mucosa, but it was significantly less efficient at dissemination than M. avium subsp. hominissuis. B-cell knockout (KO) mice, which lack Peyer's patches, were used to demonstrate that M. avium subsp. paratuberculosis enters the intestinal mucosa through enterocytes in the absence of M cells. In addition, the results indicated that M. avium subsp. paratuberculosis had equal abilities to cross the mucosa in both Peyer's patch and non-Peyer's patch segments of normal mice. M. avium subsp. paratuberculosis was also shown to interact with epithelial cells by an alpha(5)beta(1) integrin-independent pathway. Upon translocation, dendritic cells ingest M. avium subsp. paratuberculosis, but this process does not lead to efficient dissemination of the infection. In summary, M. avium subsp. paratuberculosis interacts with the intestinal mucosa by crossing both Peyer's patches and non-Peyer's patch areas but does not translocate or disseminate efficiently.
Figures

Similar articles
-
Mycobacterium avium subsp. paratuberculosis enters the small intestinal mucosa of goat kids in areas with and without Peyer's patches as demonstrated with the everted sleeve method.Comp Immunol Microbiol Infect Dis. 2005 May;28(3):223-30. doi: 10.1016/j.cimid.2005.01.004. Comp Immunol Microbiol Infect Dis. 2005. PMID: 15857661
-
Immunoperoxidase studies of cell mediated immune effector cell populations in early Mycobacterium avium subsp. paratuberculosis infection in sheep.Vet Immunol Immunopathol. 2004 Feb;97(3-4):149-62. doi: 10.1016/j.vetimm.2003.09.001. Vet Immunol Immunopathol. 2004. PMID: 14741134
-
Marked Differences in Mucosal Immune Responses Induced in Ileal versus Jejunal Peyer's Patches to Mycobacterium avium subsp. paratuberculosis Secreted Proteins following Targeted Enteric Infection in Young Calves.PLoS One. 2016 Jul 7;11(7):e0158747. doi: 10.1371/journal.pone.0158747. eCollection 2016. PLoS One. 2016. PMID: 27387969 Free PMC article.
-
Establishment of Mycobacterium avium subsp. paratuberculosis infection in the intestine of ruminants.Adv Drug Deliv Rev. 2004 Apr 19;56(6):819-34. doi: 10.1016/j.addr.2003.10.032. Adv Drug Deliv Rev. 2004. PMID: 15063592 Review.
-
No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis.Infect Immun. 2013 Nov;81(11):3960-5. doi: 10.1128/IAI.00575-13. Epub 2013 Aug 12. Infect Immun. 2013. PMID: 23940208 Free PMC article. Review.
Cited by
-
Modeling Paratuberculosis in Laboratory Animals, Cells, or Tissues: A Focus on Their Applications for Pathogenesis, Diagnosis, Vaccines, and Therapy Studies.Animals (Basel). 2023 Nov 17;13(22):3553. doi: 10.3390/ani13223553. Animals (Basel). 2023. PMID: 38003170 Free PMC article. Review.
-
Mycobacterium avium subsp. paratuberculosis Virulence: A Review.Microorganisms. 2021 Dec 19;9(12):2623. doi: 10.3390/microorganisms9122623. Microorganisms. 2021. PMID: 34946224 Free PMC article. Review.
-
Genome sequencing of ovine isolates of Mycobacterium avium subspecies paratuberculosis offers insights into host association.BMC Genomics. 2012 Mar 12;13:89. doi: 10.1186/1471-2164-13-89. BMC Genomics. 2012. PMID: 22409516 Free PMC article.
-
Inhibition of Escherichia coli invasion into bovine mammary epithelial cells previously infected by Mycobacterium avium subsp. paratuberculosis.Vet Q. 2020 Dec;40(1):43-50. doi: 10.1080/01652176.2020.1716278. Vet Q. 2020. PMID: 31939335 Free PMC article.
-
Weaning differentially affects the maturation of piglet peripheral blood and jejunal Peyer's patches.Sci Rep. 2022 Jan 31;12(1):1604. doi: 10.1038/s41598-022-05707-9. Sci Rep. 2022. PMID: 35102264 Free PMC article.
References
-
- Alonso-Hearn, M., D. Patel, L. Danelishvili, L. Meunier-Goddik, and L. E. Bermudez. 2008. The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42. Infect. Immun. 76:170-178. - PMC - PubMed
-
- Bermudez, L. E., M. Petrofsky, P. Kolonoski, and L. S. Young. 1992. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J. Infect. Dis. 165:75-79. - PubMed
-
- Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. - PMC - PubMed
-
- Chacon, O., L. E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58:329-363. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

