c-Fos proteasomal degradation is activated by a default mechanism, and its regulation by NAD(P)H:quinone oxidoreductase 1 determines c-Fos serum response kinetics
- PMID: 20498278
- PMCID: PMC2916405
- DOI: 10.1128/MCB.00899-09
c-Fos proteasomal degradation is activated by a default mechanism, and its regulation by NAD(P)H:quinone oxidoreductase 1 determines c-Fos serum response kinetics
Abstract
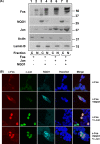
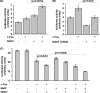
The short-lived proto-oncoprotein c-Fos is a component of the activator protein 1 (AP-1) transcription factor. A large region of c-Fos is intrinsically unstructured and susceptible to a recently characterized proteasomal ubiquitin-independent degradation (UID) pathway. UID is active by a default mechanism that is inhibited by NAD(P)H:quinone oxidoreductase 1 (NQO1), a 20S proteasome gatekeeper. Here, we show that NQO1 binds and induces robust c-Fos accumulation by blocking the UID pathway. c-Jun, a partner of c-Fos, also protects c-Fos from proteasomal degradation by default. Our findings suggest that NQO1 protects monomeric c-Fos from proteasomal UID, a function that is fulfilled later by c-Jun. We show that this process regulates c-Fos homeostasis (proteostasis) in response to serum stimulation, phosphorylation, nuclear translocation, and transcription activity. In addition, we show that NQO1 is important to ensure immediate c-Fos accumulation in response to serum, since a delayed response was observed under low NQO1 expression. These data suggest that in vivo, protein unstructured regions determine the kinetics and the homeostasis of regulatory proteins. Our data provide evidence for another layer of regulation of key regulatory proteins that functions at the level of protein degradation and is designed to ensure optimal formation of functional complexes such as AP-1.
Figures






Similar articles
-
Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1.Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13125-30. doi: 10.1073/pnas.202480499. Epub 2002 Sep 13. Proc Natl Acad Sci U S A. 2002. PMID: 12232053 Free PMC article.
-
Jun and Fos regulation of NAD(P)H: quinone oxidoreductase gene expression.Pharmacogenetics. 1994 Feb;4(1):1-10. doi: 10.1097/00008571-199402000-00001. Pharmacogenetics. 1994. PMID: 8004128 Review.
-
A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73.Genes Dev. 2005 Feb 1;19(3):316-21. doi: 10.1101/gad.319905. Genes Dev. 2005. PMID: 15687255 Free PMC article.
-
NRH:quinone oxidoreductase 2 and NAD(P)H:quinone oxidoreductase 1 protect tumor suppressor p53 against 20s proteasomal degradation leading to stabilization and activation of p53.Cancer Res. 2007 Jun 1;67(11):5380-8. doi: 10.1158/0008-5472.CAN-07-0323. Cancer Res. 2007. Retraction in: Cancer Res. 2015 Feb 1;75(3):615. doi: 10.1158/0008-5472.CAN-14-3458. PMID: 17545619 Retracted.
-
Degradation of cellular and viral Fos proteins.Biochimie. 2001 Mar-Apr;83(3-4):357-62. doi: 10.1016/s0300-9084(01)01243-3. Biochimie. 2001. PMID: 11295497 Review.
Cited by
-
Protection of c-Fos from autophagic degradation by PRMT1-mediated methylation fosters gastric tumorigenesis.Int J Biol Sci. 2023 Jul 15;19(12):3640-3660. doi: 10.7150/ijbs.85126. eCollection 2023. Int J Biol Sci. 2023. PMID: 37564212 Free PMC article.
-
FOS-ANKH and FOS-RUNX2 Fusion Genes in Osteoblastoma.Cancer Genomics Proteomics. 2020 Mar-Apr;17(2):161-168. doi: 10.21873/cgp.20176. Cancer Genomics Proteomics. 2020. PMID: 32108038 Free PMC article.
-
A Signaling Network Controlling Androgenic Repression of c-Fos Protein in Prostate Adenocarcinoma Cells.J Biol Chem. 2016 Mar 11;291(11):5512-5526. doi: 10.1074/jbc.M115.694877. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786102 Free PMC article.
-
Insights into the cellular function and mechanism of action of quinone reductase 2 (NQO2).Biochem J. 2025 Mar 11;482(6):309-24. doi: 10.1042/BCJ20240103. Biochem J. 2025. PMID: 40071447 Free PMC article. Review.
-
Compromising the 19S proteasome complex protects cells from reduced flux through the proteasome.Elife. 2015 Sep 1;4:e08467. doi: 10.7554/eLife.08467. Elife. 2015. PMID: 26327695 Free PMC article.
References
-
- Ahn, K. S., G. Sethi, A. K. Jain, A. K. Jaiswal, and B. B. Aggarwal. 2006. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 281:19798-19808. - PubMed
-
- Asher, G., Z. Bercovich, P. Tsvetkov, Y. Shaul, and C. Kahana. 2005. 20S proteasomal degradation of ornithine decarboxylase is regulated by NQO1. Mol. Cell 17:645-655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
