Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope
- PMID: 20498311
- PMCID: PMC2916299
- DOI: 10.1128/AAC.00254-10
Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope
Abstract
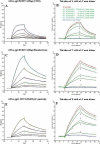
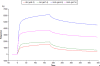
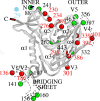
The lectin actinohivin (AH) is a monomeric carbohydrate-binding agent (CBA) with three carbohydrate-binding sites. AH strongly interacts with gp120 derived from different X4 and R5 human immunodeficiency virus (HIV) strains, simian immunodeficiency virus (SIV) gp130, and HIV type 1 (HIV-1) gp41 with affinity constants (KD) in the lower nM range. The gp120 and gp41 binding of AH is selectively reversed by (alpha1,2-mannose)3 oligosaccharide but not by alpha1,3/alpha1,6-mannose- or GlcNAc-based oligosaccharides. AH binding to gp120 prevents binding of alpha1,2-mannose-specific monoclonal antibody 2G12, and AH covers a broader epitope on gp120 than 2G12. Prolonged exposure of HIV-1-infected CEM T-cell cultures with escalating AH concentrations selects for mutant virus strains containing N-glycosylation site deletions (predominantly affecting high-mannose-type glycans) in gp120. In contrast to 2G12, AH has a high genetic barrier, since several concomitant N-glycosylation site deletions in gp120 are required to afford significant phenotypic drug resistance. AH is endowed with broadly neutralizing activity against laboratory-adapted HIV strains and a variety of X4 and/or R5 HIV-1 clinical clade isolates and blocks viral entry within a narrow concentration window of variation (approximately 5-fold). In contrast, the neutralizing activity of 2G12 varied up to 1,000-fold, depending on the virus strain. Since AH efficiently prevents syncytium formation in cocultures of persistently HIV-1-infected HuT-78 cells and uninfected CD4+ T lymphocytes, inhibits dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin-mediated capture of HIV-1 and subsequent virus transmission to CD4+ T lymphocytes, does not upregulate cellular activation markers, lacks mitogenic activity, and does not induce cytokines/chemokines in peripheral blood mononuclear cell cultures, it should be considered a potential candidate drug for microbicidal use.
Figures





Similar articles
-
Pradimicin S, a highly soluble nonpeptidic small-size carbohydrate-binding antibiotic, is an anti-HIV drug lead for both microbicidal and systemic use.Antimicrob Agents Chemother. 2010 Apr;54(4):1425-35. doi: 10.1128/AAC.01347-09. Epub 2010 Jan 4. Antimicrob Agents Chemother. 2010. PMID: 20047920 Free PMC article.
-
Differences in the mannose oligomer specificities of the closely related lectins from Galanthus nivalis and Zea mays strongly determine their eventual anti-HIV activity.Retrovirology. 2011 Feb 11;8(1):10. doi: 10.1186/1742-4690-8-10. Retrovirology. 2011. PMID: 21314946 Free PMC article.
-
The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120.J Virol. 2002 Jul;76(14):7306-21. doi: 10.1128/jvi.76.14.7306-7321.2002. J Virol. 2002. PMID: 12072529 Free PMC article.
-
Inhibition of HIV entry by carbohydrate-binding proteins.Antiviral Res. 2006 Sep;71(2-3):237-47. doi: 10.1016/j.antiviral.2006.02.004. Epub 2006 Mar 9. Antiviral Res. 2006. PMID: 16569440 Review.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
Cited by
-
The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin.Mol Pharm. 2012 Sep 4;9(9):2613-25. doi: 10.1021/mp300194b. Epub 2012 Aug 21. Mol Pharm. 2012. PMID: 22827601 Free PMC article.
-
Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models.Antimicrob Agents Chemother. 2014;58(1):120-7. doi: 10.1128/AAC.01407-13. Epub 2013 Oct 21. Antimicrob Agents Chemother. 2014. PMID: 24145548 Free PMC article.
-
Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications.Evid Based Complement Alternat Med. 2017;2017:1594074. doi: 10.1155/2017/1594074. Epub 2017 Mar 7. Evid Based Complement Alternat Med. 2017. PMID: 28367220 Free PMC article. Review.
-
A strategy for phage display selection of functional domain-exchanged immunoglobulin scaffolds with high affinity for glycan targets.J Immunol Methods. 2012 Feb 28;376(1-2):150-5. doi: 10.1016/j.jim.2011.12.008. Epub 2011 Dec 31. J Immunol Methods. 2012. PMID: 22233878 Free PMC article.
-
HIV-1 gp120 as a therapeutic target: navigating a moving labyrinth.Expert Opin Ther Targets. 2015 Jun;19(6):765-83. doi: 10.1517/14728222.2015.1010513. Epub 2015 Feb 27. Expert Opin Ther Targets. 2015. PMID: 25724219 Free PMC article. Review.
References
-
- Balzarini, J., S. Hatse, K. Vermeire, K. Princen, S. Aquaro, C. F. Perno, E. De Clercq, H. Egberink, G. Vanden Mooter, W. Peumans, E. Van Damme, and D. Schols. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48:3858-3870. - PMC - PubMed
-
- Balzarini, J., K. Van Laethem, W. J. Peumans, E. J. Van Damme, A. Bolmstedt, F. Gago, and D. Schols. 2006. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 80:8411-8421. - PMC - PubMed
-
- Balzarini, J., K. Van Laethem, D. Daelemans, S. Hatse, A. Bugatti, M. Rusnati, Y. Igarashi, T. Oki, and D. Schols. 2007. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J. Virol. 81:362-373. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

