Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants
- PMID: 20498336
- PMCID: PMC2923882
- DOI: 10.1104/pp.110.157263
Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants
Abstract
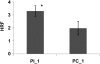
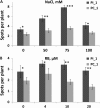
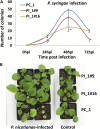
Our previous experiments showed that infection of tobacco (Nicotiana tabacum) plants with Tobacco mosaic virus (TMV) leads to an increase in homologous recombination frequency (HRF). The progeny of infected plants also had an increased rate of rearrangements in resistance gene-like loci. Here, we report that tobacco plants infected with TMV exhibited an increase in HRF in two consecutive generations. Analysis of global genome methylation showed the hypermethylated genome in both generations of plants, whereas analysis of methylation via 5-methyl cytosine antibodies demonstrated both hypomethylation and hypermethylation. Analysis of the response of the progeny of infected plants to TMV, Pseudomonas syringae, or Phytophthora nicotianae revealed a significant delay in symptom development. Infection of these plants with TMV or P. syringae showed higher levels of induction of PATHOGENESIS-RELATED GENE1 gene expression and higher levels of callose deposition. Our experiments suggest that viral infection triggers specific changes in progeny that promote higher levels of HRF at the transgene and higher resistance to stress as compared with the progeny of unstressed plants. However, data reported in these studies do not establish evidence of a link between recombination frequency and stress resistance.
Figures




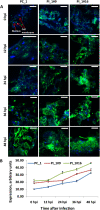
Similar articles
-
Transgenerational changes in the genome stability and methylation in pathogen-infected plants: (virus-induced plant genome instability).Nucleic Acids Res. 2007;35(5):1714-25. doi: 10.1093/nar/gkm029. Epub 2007 Feb 20. Nucleic Acids Res. 2007. PMID: 17311811 Free PMC article.
-
Identification of a Novel NtLRR-RLK and Biological Pathways That Contribute to Tolerance of TMV in Nicotiana tabacum.Mol Plant Microbe Interact. 2020 Jul;33(7):996-1006. doi: 10.1094/MPMI-12-19-0343-R. Epub 2020 May 20. Mol Plant Microbe Interact. 2020. PMID: 32196398
-
rgs-CaM Detects and Counteracts Viral RNA Silencing Suppressors in Plant Immune Priming.J Virol. 2017 Sep 12;91(19):e00761-17. doi: 10.1128/JVI.00761-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724770 Free PMC article.
-
Overexpression of NtERF5, a new member of the tobacco ethylene response transcription factor family enhances resistance to tobacco mosaic virus.Mol Plant Microbe Interact. 2004 Oct;17(10):1162-71. doi: 10.1094/MPMI.2004.17.10.1162. Mol Plant Microbe Interact. 2004. PMID: 15497409
-
Pathogen-induced systemic DNA rearrangement in plants.Trends Plant Sci. 2004 Feb;9(2):60-1. doi: 10.1016/j.tplants.2003.12.002. Trends Plant Sci. 2004. PMID: 15106587 Review.
Cited by
-
Parental methylation mediates how progeny respond to environments of parents and of progeny themselves.Ann Bot. 2022 Dec 16;130(6):883-899. doi: 10.1093/aob/mcac125. Ann Bot. 2022. PMID: 36201313 Free PMC article.
-
An epigenetic toolkit allows for diverse genome architectures in eukaryotes.Curr Opin Genet Dev. 2015 Dec;35:93-9. doi: 10.1016/j.gde.2015.10.005. Epub 2015 Nov 30. Curr Opin Genet Dev. 2015. PMID: 26649755 Free PMC article. Review.
-
Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera).PLoS Pathog. 2015 Mar 26;11(3):e1004713. doi: 10.1371/journal.ppat.1004713. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25811620 Free PMC article.
-
Changes in homologous recombination frequency in Arabidopsis thaliana plants exposed to stress depend on time of exposure during development and on duration of stress exposure.Physiol Mol Biol Plants. 2013 Oct;19(4):479-88. doi: 10.1007/s12298-013-0197-z. Physiol Mol Biol Plants. 2013. PMID: 24431516 Free PMC article.
-
Characterization, costs, cues and future perspectives of phenotypic plasticity.Ann Bot. 2022 Sep 6;130(2):131-148. doi: 10.1093/aob/mcac087. Ann Bot. 2022. PMID: 35771883 Free PMC article. Review.
References
-
- Asselbergh B, De Vleesschauwer D, Hofte M. (2008) Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21: 709–719 - PubMed
-
- Baldwin IT, Schultz JC. (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221: 277–279 - PubMed
-
- Balogun MA, Ramsay ME, Hesketh LM, Andrews N, Osborne KP, Gay NJ, Morgan-Capner P. (2002) The prevalence of hepatitis C in England and Wales. J Infect 45: 219–226 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

