High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population
- PMID: 20498355
- PMCID: PMC2890024
- DOI: 10.4049/jimmunol.0803474
High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population
Abstract
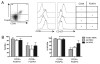
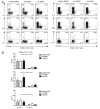
The ectonucleotidase CD39 has recently been described as being highly expressed on regulatory Foxp3(+) CD4 T cells. Through hydrolysis of proinflammatory extracellular ATP, CD39 activity represents a newly described mechanism of regulatory T cell action. We report a novel population of human CD4 T cells that express CD39 yet are Foxp3 negative. These cells produce the proinflammatory cytokines IFN-gamma and IL-17 and fail to suppress proliferation; however, they still have high ATP hydrolysis activity. In the inflammatory site in human juvenile idiopathic arthritis, the CD39(+)Foxp3(-) population is greatly increased compared with peripheral blood of patients or healthy controls. We also show that cells expressing the AMPase CD73 are less frequent in the joint than in blood. To our knowledge, this is the first study to describe and characterize CD39 function on CD4 T cells from the target site in a human autoinflammatory condition. Our data suggest that in human CD4(+) T cells from the inflamed site, CD39 can be highly expressed on two populations, one regulatory and the other of a memory phenotype.
Figures



Similar articles
-
Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype.Am J Transplant. 2010 Nov;10(11):2410-20. doi: 10.1111/j.1600-6143.2010.03291.x. Am J Transplant. 2010. PMID: 20977632 Free PMC article.
-
Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations.Am J Transplant. 2009 Oct;9(10):2303-11. doi: 10.1111/j.1600-6143.2009.02777.x. Epub 2009 Jul 28. Am J Transplant. 2009. PMID: 19656134 Free PMC article.
-
CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis.J Immunol. 2009 Dec 1;183(11):7602-10. doi: 10.4049/jimmunol.0901881. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917691
-
Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion.Eur J Immunol. 2014 Oct;44(10):2979-89. doi: 10.1002/eji.201344140. Epub 2014 Aug 25. Eur J Immunol. 2014. PMID: 24990235
-
T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine.J Immunol. 2006 Nov 15;177(10):6780-6. doi: 10.4049/jimmunol.177.10.6780. J Immunol. 2006. PMID: 17082591
Cited by
-
Generation and Function of Non-cell-bound CD73 in Inflammation.Front Immunol. 2019 Jul 26;10:1729. doi: 10.3389/fimmu.2019.01729. eCollection 2019. Front Immunol. 2019. PMID: 31404305 Free PMC article. Review.
-
Segregated regulatory CD39+CD4+ T cell function: TGF-β-producing Foxp3- and IL-10-producing Foxp3+ cells are interdependent for protection against collagen-induced arthritis.J Immunol. 2011 Nov 1;187(9):4654-66. doi: 10.4049/jimmunol.1100530. Epub 2011 Oct 3. J Immunol. 2011. PMID: 21967895 Free PMC article.
-
Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas.Neuro Oncol. 2013 Sep;15(9):1160-72. doi: 10.1093/neuonc/not067. Epub 2013 Jun 4. Neuro Oncol. 2013. PMID: 23737488 Free PMC article.
-
CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection.Transpl Immunol. 2015 Mar;32(2):76-83. doi: 10.1016/j.trim.2015.01.003. Epub 2015 Feb 7. Transpl Immunol. 2015. PMID: 25661084 Free PMC article.
-
CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients.BMC Immunol. 2013 Aug 5;14:34. doi: 10.1186/1471-2172-14-34. BMC Immunol. 2013. PMID: 23915385 Free PMC article.
References
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. - PubMed
-
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

