TGF-beta induces IL-9 production from human Th17 cells
- PMID: 20498357
- PMCID: PMC2936106
- DOI: 10.4049/jimmunol.1000356
TGF-beta induces IL-9 production from human Th17 cells
Abstract
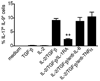
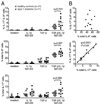
The secretion of IL-9, initially recognized as a Th2 cytokine, was recently attributed to a novel CD4 T cell subset termed Th9 in the murine system. However, IL-9 can also be secreted by mouse Th17 cells and may mediate aspects of the proinflammatory activities of Th17 cells. Here we report that IL-9 is secreted by human naive CD4 T cells in response to differentiation by Th9 (TGF-beta and IL-4) or Th17 polarizing conditions. Yet, these differentiated naive cells did not coexpress IL-17 and IL-9, unless they were repeatedly stimulated under Th17 differentiation-inducing conditions. In contrast to the naive cells, memory CD4 T cells were induced to secrete IL-9 by simply providing TGF-beta during stimulation, as neither IL-4 nor proinflammatory cytokines were required. Furthermore, the addition of TGF-beta to the Th17-inducing cytokines (IL-1beta, IL-6, IL-21, IL-23) that induce memory cells to secrete IL-17, resulted in the marked coexpression of IL-9 in IL-17 producing memory cells. The proinflammatory cytokine mediating TGF-beta-dependent coexpression of IL-9 and IL-17 was identified to be IL-1beta. Moreover, circulating monocytes were potent costimulators of IL-9 production by Th17 cells via their capacity to secrete IL-1beta. Finally, to determine whether IL-9/IL-17 coproducing CD4 cells were altered in an inflammatory condition, we examined patients with autoimmune diabetes and demonstrated that these subjects exhibit a higher frequency of memory CD4 cells with the capacity to transition into IL-9(+)IL-17(+) cells. These data demonstrate the presence of IL-17(+)IL-9(+) CD4 cells induced by IL-1beta that may play a role in human autoimmune disease.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




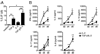


Similar articles
-
Differential induction of IL-17, IL-10, and IL-9 in human T helper cells by B7h and B7.1.Cytokine. 2013 Oct;64(1):322-30. doi: 10.1016/j.cyto.2013.05.021. Epub 2013 Jun 15. Cytokine. 2013. PMID: 23778031
-
TGF-beta promotes Th17 cell development through inhibition of SOCS3.J Immunol. 2009 Jul 1;183(1):97-105. doi: 10.4049/jimmunol.0801986. Epub 2009 Jun 17. J Immunol. 2009. PMID: 19535626 Free PMC article.
-
TLR-stimulated CD34 stem cell-derived human skin-like and monocyte-derived dendritic cells fail to induce Th17 polarization of naive T cells but do stimulate Th1 and Th17 memory responses.J Immunol. 2009 Aug 15;183(4):2242-51. doi: 10.4049/jimmunol.0900474. Epub 2009 Jul 22. J Immunol. 2009. PMID: 19625644
-
Regulation of IL-17 production in human lymphocytes.Cytokine. 2008 Feb;41(2):71-8. doi: 10.1016/j.cyto.2007.09.009. Epub 2007 Nov 5. Cytokine. 2008. PMID: 17981475 Free PMC article. Review.
-
IL-17 and Th17 Cells.Annu Rev Immunol. 2009;27:485-517. doi: 10.1146/annurev.immunol.021908.132710. Annu Rev Immunol. 2009. PMID: 19132915 Review.
Cited by
-
The development and in vivo function of T helper 9 cells.Nat Rev Immunol. 2015 May;15(5):295-307. doi: 10.1038/nri3824. Epub 2015 Apr 7. Nat Rev Immunol. 2015. PMID: 25848755 Free PMC article. Review.
-
Osteoimmunology and osteonecrosis of the femoral head.Bone Joint Res. 2022 Jan;11(1):26-28. doi: 10.1302/2046-3758.111.BJR-2021-0467.R1. Bone Joint Res. 2022. PMID: 35045723 Free PMC article. No abstract available.
-
Th9 cells: differentiation and disease.Immunol Rev. 2013 Mar;252(1):104-15. doi: 10.1111/imr.12028. Immunol Rev. 2013. PMID: 23405898 Free PMC article. Review.
-
The Th17/Treg balance and the expression of related cytokines in Uygur cervical cancer patients.Diagn Pathol. 2013 Apr 15;8:61. doi: 10.1186/1746-1596-8-61. Diagn Pathol. 2013. PMID: 23587428 Free PMC article.
-
Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity.Sci Transl Med. 2014 Jan 15;6(219):219ra8. doi: 10.1126/scitranslmed.3007828. Sci Transl Med. 2014. PMID: 24431112 Free PMC article.
References
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. - PubMed
-
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. - PubMed
-
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. - PubMed
-
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008;9:650–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

