Arabidopsis synaptotagmin SYT1, a type I signal-anchor protein, requires tandem C2 domains for delivery to the plasma membrane
- PMID: 20498364
- PMCID: PMC2906310
- DOI: 10.1074/jbc.M109.084046
Arabidopsis synaptotagmin SYT1, a type I signal-anchor protein, requires tandem C2 domains for delivery to the plasma membrane
Abstract
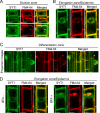
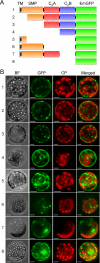
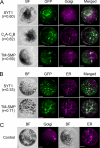
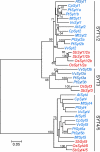
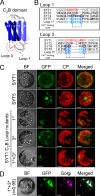
The correct localization of integral membrane proteins to subcellular compartments is important for their functions. Synaptotagmin contains a single transmembrane domain that functions as a type I signal-anchor sequence in its N terminus and two calcium-binding domains (C(2)A and C(2)B) in its C terminus. Here, we demonstrate that the localization of an Arabidopsis synaptotagmin homolog, SYT1, to the plasma membrane (PM) is modulated by tandem C2 domains. An analysis of the roots of a transformant-expressing green fluorescent protein-tagged SYT1 driven by native SYT1 promoter suggested that SYT1 is synthesized in the endoplasmic reticulum, and then delivered to the PM via the exocytotic pathway. We transiently expressed a series of truncated proteins in protoplasts, and determined that tandem C(2)A-C(2)B domains were necessary for the localization of SYT1 to the PM. The PM localization of SYT1 was greatly reduced following mutation of the calcium-binding motifs of the C(2)B domain, based on sequence comparisons with other homologs, such as endomembrane-localized SYT5. The localization of SYT1 to the PM may have been required for the functional divergence that occurred in the molecular evolution of plant synaptotagmins.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

