Destabilization of the A1 domain in von Willebrand factor dissociates the A1A2A3 tri-domain and provokes spontaneous binding to glycoprotein Ibalpha and platelet activation under shear stress
- PMID: 20498367
- PMCID: PMC2906274
- DOI: 10.1074/jbc.M110.103358
Destabilization of the A1 domain in von Willebrand factor dissociates the A1A2A3 tri-domain and provokes spontaneous binding to glycoprotein Ibalpha and platelet activation under shear stress
Abstract
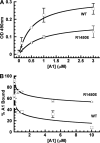
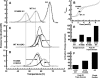
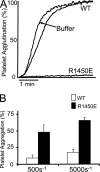
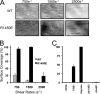
This study used recombinant A1A2A3 tri-domain proteins to demonstrate that A domain association in von Willebrand factor (VWF) regulates the binding to platelet glycoprotein Ibalpha (GPIbalpha). We performed comparative studies between wild type (WT) A1 domain and the R1450E variant that dissociates the tri-domain complex by destabilizing the A1 domain. Using urea denaturation and differential scanning calorimetry, we demonstrated the destabilization of the A1 domain structure concomitantly results in a reduced interaction among the three A domains. This dissociation results in spontaneous binding of R1450E to GPIbalpha without ristocetin with an apparent K(D) of 85 +/- 34 nm, comparable with that of WT (36 +/- 12 nm) with ristocetin. The mutant blocked 100% ristocetin-induced platelet agglutination, whereas WT failed to inhibit. The mutant enhanced shear-induced platelet aggregation at 500 and 5000 s(-1) shear rates, reaching 42 and 66%, respectively. Shear-induced platelet aggregation did not exceed 18% in the presence of WT. A1A2A3 variants were added before perfusion over a fibrin(ogen)-coated surface. At 1500 s(-1), platelets from blood containing WT adhered <10% of the surface area, whereas the mutant induced platelets to rapidly bind, covering 100% of the fibrin(ogen) surface area. Comparable results were obtained with multimeric VWF when ristocetin (0.5 mg/ml) was added to blood before perfusion. EDTA or antibodies against GPIbalpha and alphaIIbbeta3 blocked the effect of the mutant and ristocetin on platelet activation/adhesion. Therefore, the termination of A domain association within VWF in solution results in binding to GPIba and platelet activation under high shear stress.
Figures



Similar articles
-
N-terminal flanking region of A1 domain in von Willebrand factor stabilizes structure of A1A2A3 complex and modulates platelet activation under shear stress.J Biol Chem. 2012 Apr 27;287(18):14579-85. doi: 10.1074/jbc.M112.348573. Epub 2012 Mar 19. J Biol Chem. 2012. PMID: 22431729 Free PMC article. Clinical Trial.
-
Purified A2 domain of von Willebrand factor binds to the active conformation of von Willebrand factor and blocks the interaction with platelet glycoprotein Ibalpha.J Thromb Haemost. 2007 Jul;5(7):1363-70. doi: 10.1111/j.1538-7836.2007.02536.x. Epub 2007 Mar 27. J Thromb Haemost. 2007. PMID: 17389010
-
Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF.J Clin Invest. 2008 Sep;118(9):3195-207. doi: 10.1172/JCI35754. J Clin Invest. 2008. PMID: 18725999 Free PMC article.
-
The functions of the A1A2A3 domains in von Willebrand factor include multimerin 1 binding.Thromb Haemost. 2016 Jul 4;116(1):87-95. doi: 10.1160/TH15-09-0700. Epub 2016 Apr 7. Thromb Haemost. 2016. PMID: 27052467 Free PMC article.
-
Platelet physiology and thrombosis.Thromb Res. 2004;114(5-6):447-53. doi: 10.1016/j.thromres.2004.07.020. Thromb Res. 2004. PMID: 15507277 Review.
Cited by
-
Acquired von Willebrand syndrome associated with left ventricular assist device.Blood. 2016 Jun 23;127(25):3133-41. doi: 10.1182/blood-2015-10-636480. Epub 2016 May 3. Blood. 2016. PMID: 27143258 Free PMC article. Review.
-
Glycosylation sterically inhibits platelet adhesion to von Willebrand factor without altering intrinsic conformational dynamics.J Thromb Haemost. 2020 Jan;18(1):79-90. doi: 10.1111/jth.14628. Epub 2019 Sep 3. J Thromb Haemost. 2020. PMID: 31479573 Free PMC article.
-
An evaluation tool for FKBP12-dependent and -independent mTOR inhibitors using a combination of FKBP-mTOR fusion protein, DSC and NMR.Protein Eng Des Sel. 2011 Nov;24(11):811-7. doi: 10.1093/protein/gzr045. Epub 2011 Sep 6. Protein Eng Des Sel. 2011. PMID: 21900305 Free PMC article.
-
The linker between the D3 and A1 domains of vWF suppresses A1-GPIbα catch bonds by site-specific binding to the A1 domain.Protein Sci. 2013 Aug;22(8):1049-59. doi: 10.1002/pro.2294. Protein Sci. 2013. PMID: 23775931 Free PMC article.
-
Domain-specific mechanical modulation of VWF-ADAMTS13 interaction.Mol Biol Cell. 2019 Jul 22;30(16):1920-1929. doi: 10.1091/mbc.E19-01-0021. Epub 2019 May 8. Mol Biol Cell. 2019. PMID: 31067148 Free PMC article.
References
-
- Savage B., Saldívar E., Ruggeri Z. M. (1996) Cell 84, 289–297 - PubMed
-
- Sadler J. E. (1998) Annu. Rev. Biochem. 67, 395–424 - PubMed
-
- Shelton-Inloes B. B., Titani K., Sadler J. E. (1986) Biochemistry 25, 3164–3171 - PubMed
-
- Mohri H., Yoshioka A., Zimmerman T. S., Ruggeri Z. M. (1989) J. Biol. Chem. 264, 17361–17367 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

