cpSRP43 is a novel chaperone specific for light-harvesting chlorophyll a,b-binding proteins
- PMID: 20498370
- PMCID: PMC2898393
- DOI: 10.1074/jbc.C110.132746
cpSRP43 is a novel chaperone specific for light-harvesting chlorophyll a,b-binding proteins
Abstract
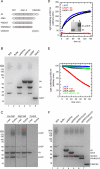
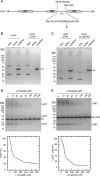
The biosynthesis of most membrane proteins is directly coupled to membrane insertion, and therefore, molecular chaperones are not required. The light-harvesting chlorophyll a,b-binding proteins (LHCPs) present a prominent exception as they are synthesized in the cytoplasm, and after import into the chloroplast, they are targeted and inserted into the thylakoid membrane. Upon arrival in the stroma, LHCPs form a soluble transit complex with the chloroplast signal recognition particle (cpSRP) consisting of an SRP54 homolog and the unique cpSRP43 composed of three chromodomains and four ankyrin repeats. Here we describe that cpSRP43 alone prevents aggregation of LHCP by formation of a complex with nanomolar affinity, whereas cpSRP54 is not required for this chaperone activity. Other stromal chaperones like trigger factor cannot replace cpSRP43, which implies that LHCPs require a specific chaperone. Although cpSRP43 does not have an ATPase activity, it can dissolve aggregates of LHCPs similar to chaperones of the Hsp104/ClpB family. We show that the LHCP-cpSRP43 interaction is predominantly hydrophobic but strictly depends on an intact DPLG motif between the second and third transmembrane region. The cpSRP43 ankyrin repeats that provide the binding site for the DPLG motif are sufficient for the chaperone function, whereas the chromodomains are dispensable. Taken together, we define cpSRP43 as a highly specific chaperone for LHCPs in addition to its established function as a targeting factor for this family of membrane proteins.
Figures
Similar articles
-
Functional analysis of the protein-interacting domains of chloroplast SRP43.J Biol Chem. 2001 Jul 6;276(27):24654-60. doi: 10.1074/jbc.M100153200. Epub 2001 Apr 16. J Biol Chem. 2001. PMID: 11306572
-
Chloroplast SRP43 acts as a chaperone for glutamyl-tRNA reductase, the rate-limiting enzyme in tetrapyrrole biosynthesis.Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):E3588-E3596. doi: 10.1073/pnas.1719645115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581280 Free PMC article.
-
Structural basis for specific substrate recognition by the chloroplast signal recognition particle protein cpSRP43.Science. 2008 Jul 11;321(5886):253-6. doi: 10.1126/science.1158640. Science. 2008. PMID: 18621669
-
Molecular mechanism of SRP-dependent light-harvesting protein transport to the thylakoid membrane in plants.Photosynth Res. 2018 Dec;138(3):303-313. doi: 10.1007/s11120-018-0544-6. Epub 2018 Jun 28. Photosynth Res. 2018. PMID: 29956039 Free PMC article. Review.
-
Component interactions, regulation and mechanisms of chloroplast signal recognition particle-dependent protein transport.Eur J Cell Biol. 2010 Dec;89(12):965-73. doi: 10.1016/j.ejcb.2010.06.020. Epub 2010 Aug 14. Eur J Cell Biol. 2010. PMID: 20709425 Review.
Cited by
-
Evolution from the prokaryotic to the higher plant chloroplast signal recognition particle: the signal recognition particle RNA is conserved in plastids of a wide range of photosynthetic organisms.Plant Cell. 2012 Dec;24(12):4819-36. doi: 10.1105/tpc.112.102996. Epub 2012 Dec 28. Plant Cell. 2012. PMID: 23275580 Free PMC article.
-
Simultaneous CAS9 editing of cpSRP43, LHCA6, and LHCA7 in Picochlorum celeri lowers chlorophyll levels and improves biomass productivity.Plant Direct. 2023 Sep 12;7(9):e530. doi: 10.1002/pld3.530. eCollection 2023 Sep. Plant Direct. 2023. PMID: 37711644 Free PMC article.
-
Interaction studies between the chloroplast signal recognition particle subunit cpSRP43 and the full-length translocase Alb3 reveal a membrane-embedded binding region in Alb3 protein.J Biol Chem. 2011 Oct 7;286(40):35187-95. doi: 10.1074/jbc.M111.250746. Epub 2011 Aug 8. J Biol Chem. 2011. PMID: 21832051 Free PMC article.
-
Conformational dynamics of a membrane protein chaperone enables spatially regulated substrate capture and release.Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):E1615-24. doi: 10.1073/pnas.1524777113. Epub 2016 Mar 7. Proc Natl Acad Sci U S A. 2016. PMID: 26951662 Free PMC article.
-
Lasting consequences of psyllid (Bactericera cockerelli L.) infestation on tomato defense, gene expression, and growth.BMC Plant Biol. 2021 Feb 24;21(1):114. doi: 10.1186/s12870-021-02876-z. BMC Plant Biol. 2021. PMID: 33627099 Free PMC article.
References
-
- Hartl F. U., Hayer-Hartl M. (2009) Nat. Struct. Mol. Biol. 16, 574–581 - PubMed
-
- Kramer G., Boehringer D., Ban N., Bukau B. (2009) Nat. Struct. Mol. Biol. 16, 589–597 - PubMed
-
- Grudnik P., Bange G., Sinning I. (2009) Biol. Chem. 390, 775–782 - PubMed
-
- Cross B. C., Sinning I., Luirink J., High S. (2009) Nat. Rev. Mol. Cell Biol. 10, 255–264 - PubMed
-
- Luirink J., von Heijne G., Houben E., de Gier J. W. (2005) Annu. Rev. Microbiol. 59, 329–355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

