Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth
- PMID: 20498373
- PMCID: PMC2911278
- DOI: 10.1074/jbc.M109.098301
Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth
Abstract
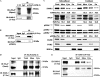
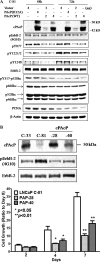
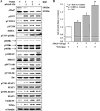
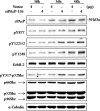
Cellular prostatic acid phosphatase (cPAcP), an authentic tyrosine phosphatase, is proposed to function as a negative growth regulator of prostate cancer (PCa) cells in part through its dephosphorylation of ErbB-2. Nevertheless, the direct interaction between cPAcP and ErbB-2 has not been shown nor the specific dephosphorylation site of ErbB-2 by cPAcP. In this report, our data show that the phosphorylation level of ErbB-2 primarily at Tyr(1221/2) correlates with the growth rate of both LNCaP and MDA PCa2b human PCa cells. Further, cPAcP reciprocally co-immunoprecipitated with ErbB-2 in a non-permissive growth condition. Expression of wild type cPAcP, but not inactive mutant, by cDNA in cPAcP-null LNCaP C-81 cells results in decreased tyrosine phosphorylation of ErbB-2 including Tyr(1221/2). Concurrently, Tyr(317) phosphorylation of p52(Shc), proliferating cell nuclear antigen expression, and cell growth are decreased in these cells. Conversely, decreased cPAcP expression by short hairpin RNA in LNCaP C-33 cells was associated with elevated phosphorylation of ErbB-2 initially at Tyr(1221/2). Its downstream p52(Shc), ERK1/2, Akt, Src, STAT-3, and STAT-5 were activated, and cell proliferation, proliferating cell nuclear antigen, and cyclin D1 expression were increased. Stable subclones of C-33 cells by small interfering PAcP had elevated Tyr(1221/2) phosphorylation of ErbB-2 and exhibited androgen-independent growth and increased tumorigenicity in xenograft female animals. In summary, our data together indicate that in prostate epithelia, cPAcP interacts with and dephosphorylates ErbB-2 primarily at Tyr(1221/2) and hence blocks downstream signaling, leading to reduced cell growth. In PCa cells, decreased cPAcP expression is associated with androgen-independent cell proliferation and tumorigenicity as seen in advanced hormone-refractory prostate carcinomas.
Figures

Similar articles
-
ErbB-2 signaling plays a critical role in regulating androgen-sensitive and castration-resistant androgen receptor-positive prostate cancer cells.Cell Signal. 2015 Nov;27(11):2261-71. doi: 10.1016/j.cellsig.2015.08.002. Epub 2015 Aug 6. Cell Signal. 2015. PMID: 26257301 Free PMC article.
-
Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer.Endocr Relat Cancer. 2005 Dec;12(4):805-22. doi: 10.1677/erc.1.00950. Endocr Relat Cancer. 2005. PMID: 16322323 Review.
-
Suppression of LNCaP prostate cancer xenograft tumors by a prostate-specific protein tyrosine phosphatase, prostatic acid phosphatase.Prostate. 2003 Jun 1;55(4):247-58. doi: 10.1002/pros.10240. Prostate. 2003. PMID: 12712404
-
Tyrosine phosphorylation of c-ErbB-2 is regulated by the cellular form of prostatic acid phosphatase in human prostate cancer cells.J Biol Chem. 1998 Aug 21;273(34):22096-104. doi: 10.1074/jbc.273.34.22096. J Biol Chem. 1998. PMID: 9705354
-
Cellular prostatic acid phosphatase, a PTEN-functional homologue in prostate epithelia, functions as a prostate-specific tumor suppressor.Biochim Biophys Acta. 2014 Aug;1846(1):88-98. doi: 10.1016/j.bbcan.2014.04.006. Epub 2014 Apr 18. Biochim Biophys Acta. 2014. PMID: 24747769 Free PMC article. Review.
Cited by
-
Screening and characterization of a novel RNA aptamer that specifically binds to human prostatic acid phosphatase and human prostate cancer cells.Mol Cells. 2015;38(2):171-9. doi: 10.14348/molcells.2015.2272. Epub 2015 Jan 15. Mol Cells. 2015. PMID: 25591398 Free PMC article.
-
Transmembrane prostatic acid phosphatase (TMPAP) interacts with snapin and deficient mice develop prostate adenocarcinoma.PLoS One. 2013 Sep 10;8(9):e73072. doi: 10.1371/journal.pone.0073072. eCollection 2013. PLoS One. 2013. PMID: 24039861 Free PMC article.
-
Seminal plasma as a source of prostate cancer peptide biomarker candidates for detection of indolent and advanced disease.PLoS One. 2013 Jun 24;8(6):e67514. doi: 10.1371/journal.pone.0067514. Print 2013. PLoS One. 2013. PMID: 23826311 Free PMC article.
-
Androgens upregulate Cdc25C protein by inhibiting its proteasomal and lysosomal degradation pathways.PLoS One. 2013 Apr 18;8(4):e61934. doi: 10.1371/journal.pone.0061934. Print 2013. PLoS One. 2013. PMID: 23637932 Free PMC article.
-
ErbB-2 signaling plays a critical role in regulating androgen-sensitive and castration-resistant androgen receptor-positive prostate cancer cells.Cell Signal. 2015 Nov;27(11):2261-71. doi: 10.1016/j.cellsig.2015.08.002. Epub 2015 Aug 6. Cell Signal. 2015. PMID: 26257301 Free PMC article.
References
-
- Huggins C., Hodges C. V. (1972) CA Cancer J. Clin. 22, 232–240 - PubMed
-
- Seidenfeld J., Samson D. J., Hasselblad V., Aronson N., Albertsen P. C., Bennett C. L., Wilt T. J. (2000) Ann. Intern. Med. 132, 566–577 - PubMed
-
- Isaacs J. T., Isaacs W. B. (2004) Nat. Med. 10, 26–27 - PubMed
-
- Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Nat. Med. 10, 33–39 - PubMed
-
- Rahman M., Miyamoto H., Chang C. (2004) Clin. Cancer Res. 10, 2208–2219 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

