Partitioning of tRNA-dependent editing between pre- and post-transfer pathways in class I aminoacyl-tRNA synthetases
- PMID: 20498377
- PMCID: PMC2911327
- DOI: 10.1074/jbc.M110.133553
Partitioning of tRNA-dependent editing between pre- and post-transfer pathways in class I aminoacyl-tRNA synthetases
Abstract
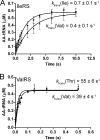
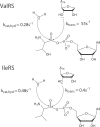
Hydrolytic editing activities are present in aminoacyl-tRNA synthetases possessing reduced amino acid discrimination in the synthetic reactions. Post-transfer hydrolysis of misacylated tRNA in class I editing enzymes occurs in a spatially separate domain inserted into the catalytic Rossmann fold, but the location and mechanisms of pre-transfer hydrolysis of misactivated amino acids have been uncertain. Here, we use novel kinetic approaches to distinguish among three models for pre-transfer editing by Escherichia coli isoleucyl-tRNA synthetase (IleRS). We demonstrate that tRNA-dependent hydrolysis of noncognate valyl-adenylate by IleRS is largely insensitive to mutations in the editing domain of the enzyme and that noncatalytic hydrolysis after release is too slow to account for the observed rate of clearing. Measurements of the microscopic rate constants for amino acid transfer to tRNA in IleRS and the related valyl-tRNA synthetase (ValRS) further suggest that pre-transfer editing in IleRS is an enzyme-catalyzed activity residing in the synthetic active site. In this model, the balance between pre-transfer and post-transfer editing pathways is controlled by kinetic partitioning of the noncognate aminoacyl-adenylate. Rate constants for hydrolysis and transfer of a noncognate intermediate are roughly equal in IleRS, whereas in ValRS transfer to tRNA is 200-fold faster than hydrolysis. In consequence, editing by ValRS occurs nearly exclusively by post-transfer hydrolysis in the editing domain, whereas in IleRS both pre- and post-transfer editing are important. In both enzymes, the rates of amino acid transfer to tRNA are similar for cognate and noncognate aminoacyl-adenylates, providing a significant contrast with editing DNA polymerases.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

