Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer
- PMID: 20498391
- PMCID: PMC2903330
- DOI: 10.1200/JCO.2009.27.0397
Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer
Abstract
Purpose: Assess efficacy and toxicity of gefitinib, an epidermal growth factor receptor (EGFR) inhibitor, added to, and in maintenance after, concurrent chemoradiotherapy (CCRT) in locally advanced head and neck cancer (LA-HNC) and correlate outcomes with EGFR gene copy number alterations.
Patients and methods: Patients with stage III to IV LA-HNC received two cycles of carboplatin/paclitaxel induction chemotherapy (IC) followed by split-course CCRT with fluorouracil, hydroxyurea, twice daily radiotherapy (FHX), and gefitinib (250 mg daily) followed by continued gefitinib for 2 years total. The primary end point was complete response (CR) rate after CCRT. EGFR gene copy number was assessed by fluorescent in situ hybridization.
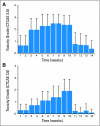
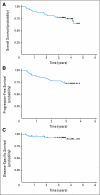
Results: Sixty-nine patients (66 with stage IV disease, 37 with oropharynx primary tumors, and 67 with performance status 0 to 1) were enrolled with a median age of 55 years. Predominant grade 3 or 4 toxicities during IC and CCRT were neutropenia (n = 20) and in-field mucositis (n = 59) and dermatitis (n = 23), respectively. CR rate after CCRT was 90%. After median follow-up of 3.5 years, 4-year overall, progression-free, and disease-specific survival rates were 74%, 72%, and 89%, respectively. To date, one patient has developed a second primary tumor in the aerodigestive tract. In 31 patients with available tissue, high EGFR gene copy number was associated with worse overall survival (P = .02).
Conclusion: Gefitinib can be administered with FHX and as maintenance therapy for at least 2 years, demonstrating CR and survival rates that compare favorably with prior experience. High EGFR gene copy number may be associated with poor outcome in patients with LA-HNC treated with this regimen.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Targeting epidermal growth factor receptor to optimize chemoradiotherapy in locally advanced head and neck cancer: has biology been taken into account?J Clin Oncol. 2011 Apr 1;29(10):e283-4; author reply e285-7. doi: 10.1200/JCO.2010.33.8087. Epub 2011 Feb 7. J Clin Oncol. 2011. PMID: 21300921 No abstract available.
References
-
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. - PubMed
-
- Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. - PubMed
-
- Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

