Volume of preclinical xenograft tumors is more accurately assessed by ultrasound imaging than manual caliper measurements
- PMID: 20498463
- PMCID: PMC2925269
- DOI: 10.7863/jum.2010.29.6.891
Volume of preclinical xenograft tumors is more accurately assessed by ultrasound imaging than manual caliper measurements
Abstract
Objective: The volume of subcutaneous xenograft tumors is an important metric of disease progression and response to therapy in preclinical drug development. Noninvasive imaging technologies suitable for measuring xenograft volume are increasingly available, yet manual calipers, which are susceptible to inaccuracy and bias, are routinely used. The goal of this study was to quantify and compare the accuracy, precision, and inter-rater variability of xenograft tumor volume assessment by caliper measurements and ultrasound imaging.
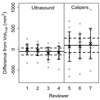
Methods: Subcutaneous xenograft tumors derived from human colorectal cancer cell lines (DLD1 and SW620) were generated in athymic nude mice. Experienced independent reviewers segmented 3-dimensional ultrasound data sets and collected manual caliper measurements resulting in tumor volumes. Imaging- and caliper-derived volumes were compared with the tumor mass, the reference standard, determined after resection. Bias, precision, and inter-rater differences were estimated for each mouse among reviewers. Bootstrapping was used to estimate mean and confidence intervals of variance components, intraclass correlation coefficients (ICCs), and confidence intervals for each source of variation.
Results: The average deviation from the true volume and inter-rater differences were significantly lower for ultrasound volumes compared with caliper volumes (P = .0005 and .001, respectively). Reviewer ICCs for ultrasound and caliper measurements were similarly low (1%), yet caliper volume variance was 1.3-fold higher than for ultrasound.
Conclusions: Ultrasound imaging more accurately, precisely, and reproducibly reflects xenograft tumor volume than caliper measurements. These data suggest that preclinical studies using the xenograft burden as a surrogate end point measured by ultrasound imaging require up to 30% fewer animals to reach statistical significance compared with analogous studies using caliper measurements.
Figures




References
-
- Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. - PubMed
-
- Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229–234. - PubMed
-
- Fiennes AG. Growth rate of human tumour xenografts measured in nude mice by in vivo cast modelling. Br J Surg. 1988;75:23–24. - PubMed
-
- Hector S, Prehn JH. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: a review. Biochim Biophys Acta. 2009;1795:117–129. - PubMed
-
- Ishimori T, Tatsumi M, Wahl RL. Tumor response assessment is more robust with sequential CT scanning than external caliper measurements. Acad Radiol. 2005;12:776–781. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 CA128323/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01 CA046413/CA/NCI NIH HHS/United States
- R01 CA140628/CA/NCI NIH HHS/United States
- K25 CA127349/CA/NCI NIH HHS/United States
- 1RC1 CA145138/CA/NCI NIH HHS/United States
- U24 CA126588/CA/NCI NIH HHS/United States
- 1P50CA128323/CA/NCI NIH HHS/United States
- RC1 CA145138/CA/NCI NIH HHS/United States
- 1R01 CA46413/CA/NCI NIH HHS/United States
- P50 95103/PHS HHS/United States
- 1R01 CA140628/CA/NCI NIH HHS/United States
- T32 EB003817/EB/NIBIB NIH HHS/United States
- U01 084239/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

