Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent
- PMID: 20498643
- PMCID: PMC2921319
- DOI: 10.1038/onc.2010.185
Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent
Abstract
We previously reported a vascular endothelial growth factor (VEGF) autocrine loop in head and neck squamous cell carcinoma (HNSCC) cell lines, supporting a role for VEGF in HNSCC tumorigenesis. Using a phosphotyrosine proteomics approach, we screened the HNSCC cell line, squamous cell carcinoma-9 for effectors of VEGFR2 signaling. A cluster of proteins involved in cell migration and invasion, including the p130Cas paralog, human enhancer of filamentation 1 (HEF1/Cas-L/Nedd9) was identified. HEF1 silencing and overexpression studies revealed a role for VEGF in regulating cell migration, invasion and matrix metalloproteinase (MMP) expression in a HEF1-dependent manner. Moreover, cells plated on extracellular matrix-coated coverslips showed enhanced invadopodia formation in response to VEGF that was HEF1-dependent. Immunolocalization revealed that HEF1 colocalized to invadopodia with MT1-MMP. Analysis of HNSCC tissue microarrays for HEF1 immunoreactivity revealed a 6.5-fold increase in the odds of having a metastasis with a high HEF1 score compared with a low HEF1 score. These findings suggest that HEF1 may be prognostic for advanced stage HNSCC. They also show for the first time that HEF1 is required for VEGF-mediated HNSCC cell migration and invasion, consistent with HEF1's recent identification as a metastatic regulator. These results support a strategy targeting VEGF:VEGFR2 in HNSCC therapeutics.
Conflict of interest statement
Figures
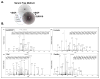
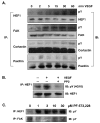
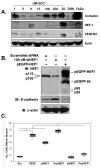
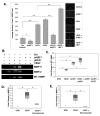

Similar articles
-
Human enhancer of filamentation 1-induced colorectal cancer cell migration: Role of serine phosphorylation and interaction with the breast cancer anti-estrogen resistance 3 protein.Int J Biochem Cell Biol. 2015 Jul;64:45-57. doi: 10.1016/j.biocel.2015.03.014. Epub 2015 Mar 25. Int J Biochem Cell Biol. 2015. PMID: 25817040
-
DCLK1-mediated regulation of invadopodia dynamics and matrix metalloproteinase trafficking drives invasive progression in head and neck squamous cell carcinoma.Mol Cancer. 2025 Feb 24;24(1):50. doi: 10.1186/s12943-025-02264-3. Mol Cancer. 2025. PMID: 39994636 Free PMC article.
-
Molecular basis for HEF1/NEDD9/Cas-L action as a multifunctional co-ordinator of invasion, apoptosis and cell cycle.Cell Biochem Biophys. 2007;48(1):54-72. doi: 10.1007/s12013-007-0036-3. Cell Biochem Biophys. 2007. PMID: 17703068 Free PMC article. Review.
-
Human enhancer of filamentation 1 Is a mediator of hypoxia-inducible factor-1alpha-mediated migration in colorectal carcinoma cells.Cancer Res. 2010 May 15;70(10):4054-63. doi: 10.1158/0008-5472.CAN-09-2110. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442290 Free PMC article.
-
A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9.Cancer Res. 2007 Oct 1;67(19):8975-9. doi: 10.1158/0008-5472.CAN-07-1328. Cancer Res. 2007. PMID: 17908996 Free PMC article. Review.
Cited by
-
Enhanced genetic instability and dasatinib sensitivity in mammary tumor cells lacking NEDD9.Cancer Res. 2010 Nov 1;70(21):8907-16. doi: 10.1158/0008-5472.CAN-10-0353. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940402 Free PMC article.
-
Aurora Kinases as Therapeutic Targets in Head and Neck Cancer.Cancer J. 2022 Sep-Oct 01;28(5):387-400. doi: 10.1097/PPO.0000000000000614. Cancer J. 2022. PMID: 36165728 Free PMC article.
-
NEDD9 depletion leads to MMP14 inactivation by TIMP2 and prevents invasion and metastasis.Mol Cancer Res. 2014 Jan;12(1):69-81. doi: 10.1158/1541-7786.MCR-13-0300. Epub 2013 Nov 7. Mol Cancer Res. 2014. PMID: 24202705 Free PMC article.
-
NEDD9 stabilizes focal adhesions, increases binding to the extra-cellular matrix and differentially effects 2D versus 3D cell migration.PLoS One. 2012;7(4):e35058. doi: 10.1371/journal.pone.0035058. Epub 2012 Apr 11. PLoS One. 2012. PMID: 22509381 Free PMC article.
-
NEDD9 overexpression predicts poor prognosis in solid cancers: a meta-analysis.Onco Targets Ther. 2019 May 28;12:4213-4222. doi: 10.2147/OTT.S205760. eCollection 2019. Onco Targets Ther. 2019. PMID: 31213839 Free PMC article. Review.
References
-
- Arold ST, Hoellerer MK, Noble MEM. The Structural Basis of Localization and Signaling by the Focal Adhesion Targeting Domain. Structure. 2002;10:319–327. - PubMed
-
- Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. - PubMed
-
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

