Effective melanoma immunotherapy in mice by the skin-depigmenting agent monobenzone and the adjuvants imiquimod and CpG
- PMID: 20498710
- PMCID: PMC2869359
- DOI: 10.1371/journal.pone.0010626
Effective melanoma immunotherapy in mice by the skin-depigmenting agent monobenzone and the adjuvants imiquimod and CpG
Abstract
Background: Presently melanoma still lacks adequate treatment options for metastatic disease. While melanoma is exceptionally challenging to standard regimens, it is suited for treatment with immunotherapy based on its immunogenicity. Since treatment-related skin depigmentation is considered a favourable prognostic sign during melanoma intervention, we here aimed at the reverse approach of directly inducing vitiligo as a shortcut to effective anti-melanoma immunity.
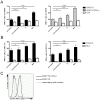
Methodology and principal findings: We developed an effective and simple to use form of immunotherapy by combining the topical skin-bleaching agent monobenzone with immune-stimulatory imiquimod cream and cytosine-guanine oligodeoxynucleotides (CpG) injections (MIC therapy). This powerful new approach promptly induced a melanoma antigen-specific immune response, which abolished subcutaneous B16.F10 melanoma growth in up to 85% of C57BL/6 mice. Importantly, this regimen induced over 100 days of tumor-free survival in up to 60% of the mice, and forcefully suppressed tumor growth upon re-challenge either 65- or 165 days after MIC treatment cessation.
Conclusions: MIC therapy is effective in eradicating melanoma, by vigilantly incorporating NK-, B- and T cells in its therapeutic effect. Based on these results, the MIC regimen presents a high-yield, low-cost and simple therapy, readily applicable in the clinic.
Conflict of interest statement
Figures
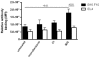



References
-
- Smith JL, Jr, Stehlin JS., Jr Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18:1399–1415. - PubMed
-
- Bart RS, Porzio NR, Kopf AW, Vilcek JT, Cheng EH, et al. Inhibition of growth of B16 murine malignant melanoma by exogenous interferon. Cancer Res. 1980;40:614–619. - PubMed
-
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

