Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis
- PMID: 20498711
- PMCID: PMC2869360
- DOI: 10.1371/journal.pone.0010627
Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that specifically affects motor neurons and leads to a progressive and ultimately fatal loss of function, resulting in death typically within 3 to 5 years of diagnosis. The disease starts with a focal centre of weakness, such as one limb, and appears to spread to other parts of the body. Mutations in superoxide dismutase 1 (SOD1) are known to cause disease and it is generally accepted they lead to pathology not by loss of enzymatic activity but by gain of some unknown toxic function(s). Although different mutations lead to varying tendencies of SOD1 to aggregate, we suggest abnormal proteins share a common misfolding pathway that leads to the formation of amyloid fibrils.
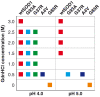
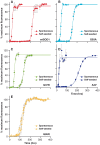
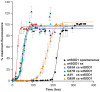
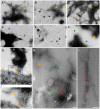
Methodology/principal findings: Here we demonstrate that misfolding of superoxide dismutase 1 leads to the formation of amyloid fibrils associated with seeding activity, which can accelerate the formation of new fibrils in an autocatalytic cascade. The time limiting event is nucleation to form a stable protein "seed" before a rapid linear polymerisation results in amyloid fibrils analogous to other protein misfolding disorders. This phenomenon was not confined to fibrils of recombinant protein as here we show, for the first time, that spinal cord homogenates obtained from a transgenic mouse model that overexpresses mutant human superoxide dismutase 1 (the TgSOD1(G93A) mouse) also contain amyloid seeds that accelerate the formation of new fibrils in both wildtype and mutant SOD1 protein in vitro.
Conclusions/significance: These findings provide new insights into ALS disease mechanism and in particular a mechanism that could account for the spread of pathology throughout the nervous system. This model of disease spread, which has analogies to other protein misfolding disorders such as prion disease, also suggests it may be possible to design assays for therapeutics that can inhibit fibril propagation and hence, possibly, disease progression.
Conflict of interest statement
Figures

References
-
- Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009;10:769–782. - PubMed
-
- Ince PG, Lowe J, Shaw PJ. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol. 1998;24:104–117. - PubMed
-
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. - PubMed
-
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

