R-flurbiprofen reduces neuropathic pain in rodents by restoring endogenous cannabinoids
- PMID: 20498712
- PMCID: PMC2869361
- DOI: 10.1371/journal.pone.0010628
R-flurbiprofen reduces neuropathic pain in rodents by restoring endogenous cannabinoids
Abstract
Background: R-flurbiprofen, one of the enantiomers of flurbiprofen racemate, is inactive with respect to cyclooxygenase inhibition, but shows analgesic properties without relevant toxicity. Its mode of action is still unclear.
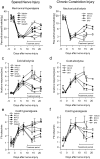
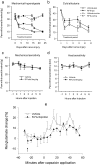
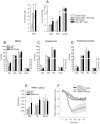
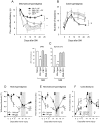
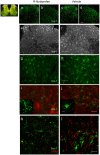
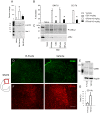
Methodology/principal findings: We show that R-flurbiprofen reduces glutamate release in the dorsal horn of the spinal cord evoked by sciatic nerve injury and thereby alleviates pain in sciatic nerve injury models of neuropathic pain in rats and mice. This is mediated by restoring the balance of endocannabinoids (eCB), which is disturbed following peripheral nerve injury in the DRGs, spinal cord and forebrain. The imbalance results from transcriptional adaptations of fatty acid amide hydrolase (FAAH) and NAPE-phospholipase D, i.e. the major enzymes involved in anandamide metabolism and synthesis, respectively. R-flurbiprofen inhibits FAAH activity and normalizes NAPE-PLD expression. As a consequence, R-Flurbiprofen improves endogenous cannabinoid mediated effects, indicated by the reduction of glutamate release, increased activity of the anti-inflammatory transcription factor PPARgamma and attenuation of microglia activation. Antinociceptive effects are lost by combined inhibition of CB1 and CB2 receptors and partially abolished in CB1 receptor deficient mice. R-flurbiprofen does however not cause changes of core body temperature which is a typical indicator of central effects of cannabinoid-1 receptor agonists.
Conclusion: Our results suggest that R-flurbiprofen improves the endogenous mechanisms to regain stability after axonal injury and to fend off chronic neuropathic pain by modulating the endocannabinoid system and thus constitutes an attractive, novel therapeutic agent in the treatment of chronic, intractable pain.
Conflict of interest statement
Figures

Similar articles
-
Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats.Eur J Neurosci. 2005 Jul;22(2):371-9. doi: 10.1111/j.1460-9568.2005.04206.x. Eur J Neurosci. 2005. PMID: 16045490
-
Anandamide deficiency and heightened neuropathic pain in aged mice.Neuropharmacology. 2013 Aug;71:204-15. doi: 10.1016/j.neuropharm.2013.03.021. Epub 2013 Apr 15. Neuropharmacology. 2013. PMID: 23597506
-
Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain.Mol Pain. 2010 Mar 17;6:16. doi: 10.1186/1744-8069-6-16. Mol Pain. 2010. PMID: 20236533 Free PMC article.
-
[The cannabinoid system and pain: towards new drugs?].J Soc Biol. 2009;203(1):99-106. doi: 10.1051/jbio:2009002. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358815 Review. French.
-
Targeting the cannabinoid system for pain relief?Acta Anaesthesiol Taiwan. 2013 Dec;51(4):161-70. doi: 10.1016/j.aat.2013.10.004. Epub 2013 Dec 25. Acta Anaesthesiol Taiwan. 2013. PMID: 24529672 Review.
Cited by
-
Inhibition of endocannabinoid metabolism by the metabolites of ibuprofen and flurbiprofen.PLoS One. 2014 Jul 25;9(7):e103589. doi: 10.1371/journal.pone.0103589. eCollection 2014. PLoS One. 2014. PMID: 25061885 Free PMC article.
-
Endocannabinoids as Guardians of Metastasis.Int J Mol Sci. 2016 Feb 10;17(2):230. doi: 10.3390/ijms17020230. Int J Mol Sci. 2016. PMID: 26875980 Free PMC article. Review.
-
Low brain endocannabinoids associated with persistent non-goal directed nighttime hyperactivity after traumatic brain injury in mice.Sci Rep. 2020 Sep 10;10(1):14929. doi: 10.1038/s41598-020-71879-x. Sci Rep. 2020. PMID: 32913220 Free PMC article.
-
Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation.Nat Neurosci. 2013 Sep;16(9):1291-8. doi: 10.1038/nn.3480. Epub 2013 Aug 4. Nat Neurosci. 2013. PMID: 23912944 Free PMC article.
-
Therapeutic Effect of the Substrate-Selective COX-2 Inhibitor IMMA in the Animal Model of Chronic Constriction Injury.Front Pharmacol. 2018 Dec 18;9:1481. doi: 10.3389/fphar.2018.01481. eCollection 2018. Front Pharmacol. 2018. PMID: 30618769 Free PMC article.
References
-
- Ma W, Quirion R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin Ther Targets. 2007;11:307–320. - PubMed
-
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. Faseb J. 2001;15:2057–2072. - PubMed
-
- Geisslinger G, Muth-Selbach U, Coste O, Vetter G, Schrodter A, et al. Inhibition of noxious stimulus-induced spinal prostaglandin E2 release by flurbiprofen enantiomers: a microdialysis study. J Neurochem. 2000;74:2094–2100. - PubMed
-
- Tegeder I, Niederberger E, Israr E, Guhring H, Brune K, et al. Inhibition of NF-kappaB and AP-1 activation by R- and S-flurbiprofen. Faseb J. 2001;15:595–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

