Higher expression of the heterogeneous nuclear ribonucleoprotein k in melanoma
- PMID: 20499280
- PMCID: PMC2943057
- DOI: 10.1245/s10434-010-1121-1
Higher expression of the heterogeneous nuclear ribonucleoprotein k in melanoma
Abstract
Background: The heterogeneous nuclear ribonucleoprotein (hnRNP) K is an essential RNA and DNA binding protein involved in gene expression and signal transduction. The role of hnRNP K in cancer is relatively understudied. However, several cellular functions strongly indicate that hnRNP K is involved in tumorigenesis. Oncogenes c-Src, c-myc, and eIF4E are regulated by hnRNP K. We have shown an increased cytoplasmic hnRNP K in pancreatic cancer. In the present study, we investigated the altered expression of hnRNP K protein and its correlation with p-ERK in melanoma using human melanoma cell lines and tissue microarray.
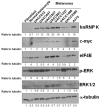
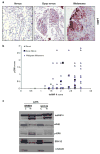
Materials and methods: The protein levels of hnRNP K and p-ERK in 8 human melanoma cell lines and a melanoma progression tissue microarray containing 80 melanoma, 23 dysplastic nevi, and 14 benign nevi specimens were analyzed using Western blot and immunohistochemistry analysis. hnRNP K was knocked down by siRNA, and its effect on melanoma cells was assessed.
Results: We showed a higher hnRNP K protein level in both melanoma cell lines and melanoma tissue specimens, which correlated with a higher c-myc expression. An increase in the cytoplasmic hnRNP K and eIF4E protein levels in melanoma cells is also seen. p-ERK level was also higher in dysplastic nevi and melanoma tissues, but did not correlate with hnRNP K protein level. We then demonstrated that knocking down of hnRNP K by siRNA inhibited melanoma cell growth and colony formation, as well as c-myc expression.
Conclusions: hnRNP K expression correlated with melanoma and may play a role in melanoma tumorigenesis.
Figures


References
-
- Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004;26:629–38. - PubMed
-
- Klimek-Tomczak K, Wyrwicz LS, Jain S, Bomsztyk K, Ostrowski J. Characterization of hnRNP K protein-RNA interactions. J Mol Biol. 2004;342:1131–41. - PubMed
-
- Mikula M, Dzwonek A, Karczmarski J, Rubel T, Dadlez M, Wyrwicz LS, et al. Landscape of the hnRNP K protein-protein interactome. Proteomics. 2006;6:2395–406. - PubMed
-
- Baber JL, Libutti D, Levens D, Tjandra N. High precision solution structure of the C-terminal KH domain of heterogeneous nuclear ribonucleoprotein K, a c-myc transcription factor. J Mol Biol. 1999;289:949–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

