Automated electron-density sampling reveals widespread conformational polymorphism in proteins
- PMID: 20499387
- PMCID: PMC2974833
- DOI: 10.1002/pro.423
Automated electron-density sampling reveals widespread conformational polymorphism in proteins
Abstract
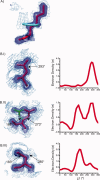
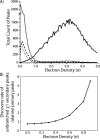
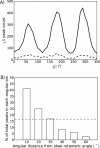
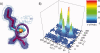
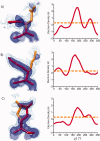
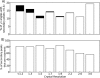
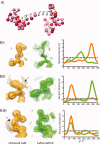
Although proteins populate large structural ensembles, X-ray diffraction data are traditionally interpreted using a single model. To search for evidence of alternate conformers, we developed a program, Ringer, which systematically samples electron density around the dihedral angles of protein side chains. In a diverse set of 402 structures, Ringer identified weak, nonrandom electron-density features that suggest of the presence of hidden, lowly populated conformations for >18% of uniquely modeled residues. Although these peaks occur at electron-density levels traditionally regarded as noise, statistically significant (P < 10(-5)) enrichment of peaks at successive rotameric chi angles validates the assignment of these features as unmodeled conformations. Weak electron density corresponding to alternate rotamers also was detected in an accurate electron density map free of model bias. Ringer analysis of the high-resolution structures of free and peptide-bound calmodulin identified shifts in ensembles and connected the alternate conformations to ligand recognition. These results show that the signal in high-resolution electron density maps extends below the traditional 1 sigma cutoff, and crystalline proteins are more polymorphic than current crystallographic models. Ringer provides an objective, systematic method to identify previously undiscovered alternate conformations that can mediate protein folding and function.
Figures
Comment in
-
Peripatetic proteins.Protein Sci. 2010 Jul;19(7):1279-80. doi: 10.1002/pro.422. Protein Sci. 2010. PMID: 20499369 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

