Fluvastatin in the first-line therapy of acute coronary syndrome: results of the multicenter, randomized, double-blind, placebo-controlled trial (the FACS-trial)
- PMID: 20500832
- PMCID: PMC2886041
- DOI: 10.1186/1745-6215-11-61
Fluvastatin in the first-line therapy of acute coronary syndrome: results of the multicenter, randomized, double-blind, placebo-controlled trial (the FACS-trial)
Abstract
Background: Statins have been proved to be effective in reduction of mortality and morbidity when started in the early secondary prevention in stabilized patients after acute coronary syndrome (ACS). The safety and efficacy of statin administration directly in the first-line therapy in unstable ACS patients is not clear. The aim of our study was, therefore, to assess the effect of statin treatment initiated immediately at hospital admission of patients with ACS.
Methods: The trial was stopped prematurely after enrollment of one hundred and fifty-six patients with ACS that were randomized at admission to fluvastatin 80 mg (N = 78) or placebo (N = 78). Study medication was administered immediately after randomization and then once daily for 30 days; all patients were then encouraged to continue in open-label statin therapy and at the end of one-year follow-up 75% in the fluvastatin group and 78% in the placebo group were on statin therapy.
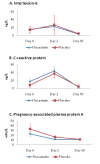
Results: We did not demonstrate any difference between groups in the level of C-reactive protein, interleukin 6, and pregnancy-associated plasma protein A on Day 2 and Day 30 (primary endpoint). Fluvastatin-therapy, however, significantly reduced one-year occurrence of major adverse cardiovascular events (11.5% vs. 24.4%, odds ratio (OR) 0.40, 95% CI 0.17-0.95, P = 0.038). This difference was caused mainly by reduction of recurrent symptomatic ischemia (7.7% vs. 20.5%, OR 0.32, 95% CI 0.12-0.88, P = 0.037).
Conclusions: This study failed to prove the effect of fluvastatin given as first-line therapy of ACS on serum markers of inflammation and plaque instability. Fluvastatin therapy was, however, safe and it may reduce cardiovascular event rate that supports immediate use of a statin in patients admitted for ACS.
Trial registration: NCT00171275.
Figures

References
-
- Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. Jama. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. - DOI - PubMed
-
- de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. Jama. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. - DOI - PubMed
-
- Hayashidani S, Tsutsui H, Shiomi T, Suematsu N, Kinugawa S, Ide T, Wen J, Takeshita A. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–873. doi: 10.1161/hc0702.104164. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

