Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats
- PMID: 20500834
- PMCID: PMC2911891
- DOI: 10.1186/ar3034
Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats
Abstract
Introduction: Toll-like receptors (TLRs) are involved in both innate and adaptive immune responses and are likely to play a complex role in the pathogenesis of human rheumatoid arthritis (RA) and experimental arthritis. The objective of this study was to identify the key TLR in pristane-induced arthritis (PIA), a rat model for RA, and to clarify its roles in the initiation and maintenance of arthritis.
Methods: Arthritis in DA rats was induced by pristane and the severity was evaluated by macroscopic and microscopic score systems. Spleen TLR and cytokine expression was detected at different time points by real-time polymerase chain reaction (PCR) and flow cytometry. Polyinosine-polycytidylic acid (polyI:C, a ligand of TLR3) or TLR3 specific short-hairpin RNA plasmid for RNA interference was administrated to PIA rats in vivo. Serum nitrogen oxide concentration was determined by Griess method, and tumor necrosis factor alpha (TNF-alpha) was determined by L929 biotest. In splenic macrophages, TLR3 expression was measured by flow cytometry. A rat macrophage cell line (NR8383) was stimulated by pristane, and anti-TLR3 antibody were used to block TLR3 pathway. TLR3 and cytokine expression in NR8383 were detected by real-time PCR.
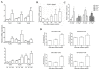
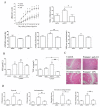
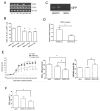
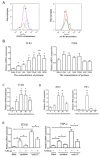
Results: By screening the TLR expression profile in spleen of DA rats after pristane injection, we found that TLR3 was the most early and prominently upregulated TLR. Both TLR3 mRNA and protein expression of spleen were upregulated at 6 and 26 days after pristane injection. Furthermore, administration of polyI:C exacerbated, whereas RNA interference targeting TLR3 ameliorated, the arthritis. Particularly, TLR3 expression was induced in splenic macrophages of PIA rats, and also in the NR8383 cell line after pristane stimulation in a dose- and time- dependent manner. Upregulation of interferon beta (IFN-beta) and TNF-alpha by pristane stimulation was blocked by anti-TLR3 antibody in NR8383.
Conclusions: TLR3 plays a pivotal role in the initiation and development of PIA which may dependent on macrophage. These findings are useful to understand the pathogenesis of RA and may provide an intriguing therapeutic opportunity for RA.
Figures
Similar articles
-
Arthritis is associated with T-cell-induced upregulation of Toll-like receptor 3 on synovial fibroblasts.Arthritis Res Ther. 2011 Jun 27;13(3):R103. doi: 10.1186/ar3384. Arthritis Res Ther. 2011. PMID: 21708001 Free PMC article.
-
MicroRNA-26a negatively regulates toll-like receptor 3 expression of rat macrophages and ameliorates pristane induced arthritis in rats.Arthritis Res Ther. 2014 Jan 14;16(1):R9. doi: 10.1186/ar4435. Arthritis Res Ther. 2014. PMID: 24423102 Free PMC article.
-
Overexpression of toll-like receptor 3 in spleen is associated with experimental arthritis in rats.Scand J Immunol. 2012 Sep;76(3):263-70. doi: 10.1111/j.1365-3083.2012.02724.x. Scand J Immunol. 2012. PMID: 22702720
-
Toll-like receptor 3 involvement in vascular function.Eur J Pharmacol. 2024 Sep 15;979:176842. doi: 10.1016/j.ejphar.2024.176842. Epub 2024 Jul 19. Eur J Pharmacol. 2024. PMID: 39033837 Review.
-
Toll-Like Receptor 3 in Cardiovascular Diseases.Heart Lung Circ. 2022 Jul;31(7):e93-e109. doi: 10.1016/j.hlc.2022.02.012. Epub 2022 Mar 30. Heart Lung Circ. 2022. PMID: 35367134 Review.
Cited by
-
Arsenic album 30C exhibits crystalline nano structure of arsenic trioxide and modulates innate immune markers in murine macrophage cell lines.Sci Rep. 2024 Jan 7;14(1):745. doi: 10.1038/s41598-024-51319-w. Sci Rep. 2024. Retraction in: Sci Rep. 2024 Aug 21;14(1):19378. doi: 10.1038/s41598-024-70287-9. PMID: 38185726 Free PMC article. Retracted.
-
A genome-wide CRISPR screen identifies regulation factors of the TLR3 signalling pathway.Innate Immun. 2020 Aug;26(6):459-472. doi: 10.1177/1753425920915507. Epub 2020 Apr 4. Innate Immun. 2020. PMID: 32248720 Free PMC article.
-
Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis.J Pers Med. 2023 Oct 29;13(11):1550. doi: 10.3390/jpm13111550. J Pers Med. 2023. PMID: 38003865 Free PMC article. Review.
-
Membrane-bound toll-like receptors are overexpressed in peripheral blood and synovial fluid mononuclear cells of enthesitis-related arthritis category of juvenile idiopathic arthritis (JIA–ERA) patients and lead to secretion of inflammatory mediators.J Clin Immunol. 2012 Jun;32(3):488-96. doi: 10.1007/s10875-011-9640-5. J Clin Immunol. 2012. PMID: 22302567
-
Investigation of the role of endosomal Toll-like receptors in murine collagen-induced arthritis reveals a potential role for TLR7 in disease maintenance.Arthritis Res Ther. 2012 Jun 12;14(3):R142. doi: 10.1186/ar3875. Arthritis Res Ther. 2012. PMID: 22691272 Free PMC article.
References
-
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/S1074-7613(02)00275-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

