Progesterone modulation of transmembrane helix-helix interactions between the alpha-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane
- PMID: 20500835
- PMCID: PMC2887865
- DOI: 10.1186/1472-6807-10-12
Progesterone modulation of transmembrane helix-helix interactions between the alpha-subunit of Na/K-ATPase and phospholipid N-methyltransferase in the oocyte plasma membrane
Abstract
Background: Progesterone binding to the surface of the amphibian oocyte initiates the meiotic divisions. Our previous studies with Rana pipiens oocytes indicate that progesterone binds to a plasma membrane site within the external loop between the M1 and M2 helices of the alpha-subunit of Na/K-ATPase, triggering a cascade of lipid second messengers and the release of the block at meiotic prophase. We have characterized this site, using a low affinity ouabain binding isoform of the alpha1-subunit.
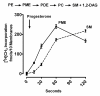
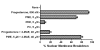
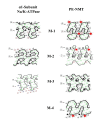
Results: Preparations of isolated plasma membranes from Rana oocytes demonstrate that physiological levels of progesterone (or the non-metabolizable progestin R5020) successively activate phosphatidylethanolamine-N-methyltransferase (PE-NMT) and sphingomyelin synthase within seconds. Inhibition of PE-NMT blocks the progesterone induction of meiosis in intact oocytes, whereas its initial product, phosphatidylmonomethylethanolamine (PME), can itself initiate meiosis in the presence of the inhibitor. Published X-ray crystallographic data on Na/K-ATPase, computer-generated 3D projections, heptad repeat analysis and hydrophobic cluster analysis of the transmembrane helices predict that hydrophobic residues L, V, V, I, F and Y of helix M2 of the alpha1-subunit interact with F, L, G, L, L and F, respectively, of helix M3 of PE-NMT.
Conclusion: We propose that progesterone binding to the first external loop of the alpha1-subunit facilitates specific helix-helix interactions between integral membrane proteins to up-regulate PE-NMT, and, that successive interactions between two or more integral plasma membrane proteins induce the signaling cascades which result in completion of the meiotic divisions.
Figures





Similar articles
-
Caveolin-Na/K-ATPase interactions: role of transmembrane topology in non-genomic steroid signal transduction.Steroids. 2012 Sep;77(11):1160-8. doi: 10.1016/j.steroids.2012.04.012. Epub 2012 May 9. Steroids. 2012. PMID: 22579740
-
Progesterone binding to the alpha1-subunit of the Na/K-ATPase on the cell surface: insights from computational modeling.Steroids. 2008 Jan;73(1):27-40. doi: 10.1016/j.steroids.2007.08.012. Epub 2007 Sep 2. Steroids. 2008. PMID: 17936318 Free PMC article.
-
The steroid-binding subunit of the Na/K-ATPase as a progesterone receptor on the amphibian oocyte plasma membrane.Steroids. 2005 Dec 15;70(14):933-45. doi: 10.1016/j.steroids.2005.07.002. Epub 2005 Sep 13. Steroids. 2005. PMID: 16165176
-
Progesterone induces meiotic division in the amphibian oocyte by releasing lipid second messengers from the plasma membrane.Steroids. 1999 Jan-Feb;64(1-2):157-67. doi: 10.1016/s0039-128x(98)00093-2. Steroids. 1999. PMID: 10323685 Review.
-
General and specific lipid-protein interactions in Na,K-ATPase.Biochim Biophys Acta. 2015 Sep;1848(9):1729-43. doi: 10.1016/j.bbamem.2015.03.012. Epub 2015 Mar 16. Biochim Biophys Acta. 2015. PMID: 25791351 Review.
Cited by
-
One-carbon metabolism during the menstrual cycle and pregnancy.PLoS Comput Biol. 2021 Dec 16;17(12):e1009708. doi: 10.1371/journal.pcbi.1009708. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34914693 Free PMC article.
-
Testis-Specific Isoform of Na+-K+ ATPase and Regulation of Bull Fertility.Int J Mol Sci. 2022 Jul 19;23(14):7936. doi: 10.3390/ijms23147936. Int J Mol Sci. 2022. PMID: 35887284 Free PMC article. Review.
-
Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase.Biochemistry. 2011 Oct 11;50(40):8664-73. doi: 10.1021/bi2009008. Epub 2011 Sep 16. Biochemistry. 2011. PMID: 21905705 Free PMC article.
-
Progesterone-induced changes in the phosphoryl potential during the meiotic divisions in amphibian oocytes: role of Na/K-ATPase.BMC Dev Biol. 2011 Nov 6;11:67. doi: 10.1186/1471-213X-11-67. BMC Dev Biol. 2011. PMID: 22054214 Free PMC article.
-
Evolution of the α-Subunit of Na/K-ATPase from Paramecium to Homo sapiens: Invariance of Transmembrane Helix Topology.J Mol Evol. 2016 May;82(4-5):183-98. doi: 10.1007/s00239-016-9732-1. Epub 2016 Mar 10. J Mol Evol. 2016. PMID: 26961431 Free PMC article.
References
-
- Blanco G, Mercer RW. Isozymes of the Na/K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275(5 Pt 2):F633–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

