A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer
- PMID: 20501622
- PMCID: PMC3035576
- DOI: 10.1158/1078-0432.CCR-10-0124
A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer
Abstract
Purpose: Given discrepancies between preclinical and clinical observations of mammalian target of rapamycin (mTOR) inhibition in prostate cancer, we sought to determine the pharmacodynamic effects of the mTOR/TORC1 inhibitor rapamycin in men with intermediate- to high-risk prostate cancer undergoing radical prostatectomy.
Experimental design: Rapamycin was given at 3 or 6 mg orally for 14 days before radical prostatectomy in men with multifocal Gleason sum > or =7 prostate cancer; 10 untreated control subjects were included. The primary outcome was inhibition of phosphorylation of ribosomal S6 in posttreatment radical prostatectomy versus pretreatment biopsy tumor tissue, evaluated using a Simon two-stage design for pharmacodynamic efficacy.
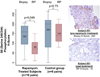
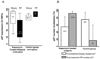
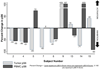
Results: Thirty-two subjects were accrued: 20 at 3 mg, 2 at 6 mg, and 10 controls. No dose-limiting toxicities were observed at 3 mg; however, two of two men enrolled at 6 mg experienced dose-limiting toxicities including thrombocytopenia and fever with grade 3 stomatitis. Adverse events observed at 3 mg included stomatitis, rash, ileus, and neutropenia. Pharmacodynamic studies showed tumor S6 phosphorylation inhibition in 50% of 10 evaluable rapamycin-treated men with sufficient paired tissue [median 58% inhibition (P = 0.049) versus 2% inhibition in controls (P = 0.75)] with no significant effect on AKT activity. We observed no change in Ki-67 or caspase-3 cleavage but noted a reduction in cytoplasmic p27 staining with increased nuclear localization with rapamycin treatment. Prostate tissue rapamycin concentrations were 3- to 4-fold higher than blood.
Conclusions: At 3 mg daily, rapamycin successfully and safely inhibited prostate cancer S6 phosphorylation and achieved relatively high prostate tissue concentrations. No effect on AKT phosphorylation, tumor proliferation, or apoptosis was observed.
Copyright 2010 AACR.
Figures


References
-
- Figlin RA, Brown E, Armstrong AJ, Akerley W, Benson AB, III, Burstein HJ, et al. NCCN Task Force Report: mTOR inhibition in solid tumors. J Natl Compr Canc Netw. 2008 Sep 6; Suppl 5:S1–S20. - PubMed
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007 May 31;356(22):2271–2281. - PubMed
-
- Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003 Jun;28(2):145–153. - PubMed
-
- Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998 Jan 15;58(2):204–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

