Simultaneous pharmacokinetics/pharmacodynamics modeling of recombinant human erythropoietin upon multiple intravenous dosing in rats
- PMID: 20501635
- PMCID: PMC2939674
- DOI: 10.1124/jpet.110.167304
Simultaneous pharmacokinetics/pharmacodynamics modeling of recombinant human erythropoietin upon multiple intravenous dosing in rats
Abstract
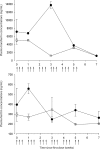
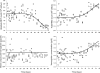
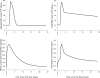
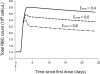
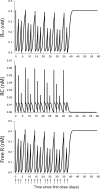
A pharmacokinetics (PK)/pharmacodynamics (PD) model was developed to describe the tolerance and rebound for reticulocyte (RET) and red blood cell (RBC) counts and the hemoglobin (Hb) concentrations in blood after repeated intravenous administrations of 1350 IU/kg of recombinant human erythropoietin (rHuEPO) in rats thrice weekly for 6 weeks. Drug concentrations were described by using a quasi-equilibrium model. The PD model consisted of a lifespan-based indirect response model (LIDR) with progenitor cells [burst colony-forming unit erythroblasts and colony-forming unit erythroblasts (CFUs)], normoblasts (NOR), RETs, and RBCs. Drug-receptor complex stimulatory effects on progenitor cells differentiation and RBC lifespan were expressed by using the E(max) model (S(max-epo) and SC(50-epo), E(max) and EC(50)). The Hb profile was indirectly modeled through a LIDR model for mean corpuscular hemoglobin (with a lifespan T(mch)) including a linear (S(max-mch)) drug stimulatory effect. The negative feedback from RBCs accounted for the time-dependent rHuEPO clearance decline. A simultaneous PK/PD fitting was performed by using MATLAB-based software. PK parameters such as equilibrium dissociation, erythropoietin receptor degradation, production, and internalization rate constants were 0.18 nM (fixed), 0.08 h(-1), 0.03 nM/h, and 2.51 h(-1), respectively. The elimination rate constant and central volume of distribution were 0.57 h(-1) and 40.63 ml/kg, respectively. CFU and NOR, RET, and RBC lifespans were 37.26 h, 17.25 h, and 30.15 days, respectively. S(max-epo) and SC(50-epo) were 7.3 and 0.47 10(-2) nM, respectively. E(max) was fixed to 1. EC(50) and SC(50-epo) were equal. S(max-mch) and T(mch) were 168.1 nM(-1) and 35.15 days, respectively. The proposed PK/PD model effectively described rHuEPO nonstationary PK and allowed physiological estimates of cell lifespans.
Figures





References
-
- Agoram B, Aoki K, Doshi S, Gegg C, Jang G, Molineux G, Narhi L, Elliott S. (2009) Investigation of the effects of altered receptor binding activity on the clearance of erythropoiesis-stimulating proteins: nonerythropoietin receptor-mediated pathways may play a major role. J Pharm Sci 98:2198–2211 - PubMed
-
- Akahane K, Tojo A, Fukamachi H, Kitamura T, Saito T, Urabe A, Takaku F. (1989) Binding of iodinated erythropoietin to rat bone marrow cells under normal and anemic conditions. Exp Hematol 17:177–182 - PubMed
-
- Archer RK, Festing MF, Riley J. (1982) Haematology of conventionally-maintained Lac:P outbred Wistar rats during the 1st year of life. Lab Anim 16:198–200 - PubMed
-
- Bogdanova A, Mihov D, Lutz H, Saam B, Gassmann M, Vogel J. (2007) Enhanced erythro-phagocytosis in polycythemic mice overexpressing erythropoietin. Blood 110:762–769 - PubMed
-
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, et al. (2002) Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med 346:469–475 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

