Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function
- PMID: 20501640
- PMCID: PMC2903904
- DOI: 10.1210/me.2010-0006
Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function
Abstract
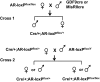
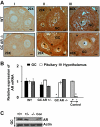
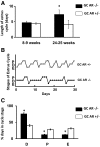
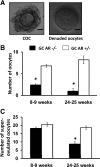
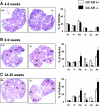
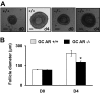
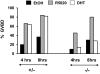
The physiological significance of androgens in female reproduction was unclear until female mice with global knockout of androgen receptor (AR) expression were found to have reduced fertility with abnormal ovarian function. However, because ARs are expressed in a myriad of reproductive tissues, including the hypothalamus, pituitary, and various ovarian cells, the role of tissue-specific ARs in regulating female fertility remained unknown. To examine the importance of ovarian ARs in female reproduction, we generated granulosa cell (GC)- and oocyte-specific AR-knockout (ARKO) mice by crossing AR-flox mice with MisRIIcre (GC-specific) or growth differentiation factor growth differentiation factor-9cre (oocyte-specific) mice. Relative to heterozygous and wild-type mice, GC-specific ARKO mice had premature ovarian failure and were subfertile, with longer estrous cycles and fewer ovulated oocytes. In addition, ovaries from GC-specific knockout mice contained more preantral and atretic follicles, with fewer antral follicles and corpus lutea. Finally, in vitro growth of follicles from GC-specific AR-null mice was slower than follicles from wild-type animals. In contrast to GC-specific AR-null mice, fertility, estrous cycles, and ovarian morphology of oocyte-specific ARKO mice were normal, although androgens no longer promoted oocyte maturation in these animals. Together, our data indicate that nearly all reproductive phenotypes observed in global ARKO mice can be explained by the lack of AR expression in GCs. These GC-specific ARs appear to promote preantral follicle growth and prevent follicular atresia; thus they are essential for normal follicular development and fertility.
Figures
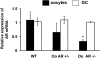
References
-
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 - PubMed
-
- Lange CA, Gioeli D, Hammes SR, Marker PC 2007 Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol 69:171–199 - PubMed
-
- Xu Y, Chen SY, Ross KN, Balk SP 2006 Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 66:7783–7792 - PubMed
-
- Hillier SG, Whitelaw PF, Smyth CD 1994 Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 100:51–54 - PubMed
-
- Simpson ER 2002 Aromatization of androgens in women: current concepts and findings. Fertil Steril 77(Suppl 4):S6–S10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

